Page 115 - 2021_06-Haematologica-web
P. 115
Bile acids reduce GvHD and preserve the GvL effect
Representative data from one of two biologically independent experiments performed with three to four technical replicates respectively. (H) Experimental model for assessing the graft-versus-ligand (GvL) response of allogeneic T cells ex vivo. BALB/c mice underwent bone marrow transplantation (BMT) as described in Figure 3C and T cells from spleens were isolated for subsequent co-culture with A20 cells on day 14 after BMT. (I) Flow cytometric quantification of dead A20 lymphoma cells co-cultured with CD4+ and CD8+ T cells re-isolated from the spleens of recipient mice on day 14 after BMT as described in (H). Representative data from one of two biologically independent experiments performed with four to five technical replicates respectively. P-values were calculated using the ordinary one-way ANOVA test with correction for multiple comparisons, ns: not significant. (J) Experimental model for assessing the GvL response in vivo. BALB/c mice underwent BMT with addi- tional injection of green fluorescent protein positive (GFP+) Ba/F3-ITD leukemia cells. Allogeneic T cells were transferred two days later and animals were treated with 200 mg/kg body weight TUDCA or vehicle for another 10 days. (K) Flow cytometric analysis of spleen and bone marrow for the percentage of GFP+ cells on day 12 after tumor injection. N numbers represent individual mice. Left panel: representative flow cytometry plots. Right panel: quantification, numbers (N) represent indi- vidual mice. P-values were calculated using the ordinary one-way ANOVA test with correction for multiple comparisons, ns: not significant.
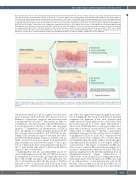
Figure 8. Tauroursodeoxycholic acid leads to a reduction of acute graft-versus-host disease. A model, in which this positive effect is achieved by two distinct mech- anisms: enhancing the viability during exposure to pro-inflammatory cytokines and reduction of antigen presentation in the intestine with a consequent decrease in apoptosis.
cytoprotective functions. UDCA is approved for the treat- ment of patients with cholestatic liver diseases based on inhibition of hepatocyte apoptosis and protection from toxic hydrophobic bile acids.26,32 These effects are linked to a stabilization of the mitochondria, reduced BAX transloca- tion and decreased cytochrome C release and subsequent apoptosis.32,33 Furthermore, the administration of TUDCA led to the inhibition of cellular damage introduced by the bile acid glycochenodeoxycholic acid (GCDCA) by pre- venting GCDCA-induced caspase-9 activation and subse- quent mitochondrial damage. Therefore, such bile acids can enable survival via protection against more hydrophobic and potentially more toxic bile acid variants.34,35 Also a pro- tection of hepatocytes from carcinogen-induced apoptosis36 and of renal tubular cells against contrast media-induced apoptosis37 has been described.
Translating these data into a preclinical BMT model, we observed that application of the bile acid TUDCA, the most potent agent in our in vitro studies, prolonged the survival of mice with aGvHD. Exogenous bile acid application was able to substantially modulate the bile acid pool. TUDCA increased to 35% of all measured bile acids in the serum and almost 60% of the bile acids measured in the ileal con-
tent. These data suggest that exogenous application is effec- tive in changing the bile acid pool and therefore probably counteracts the depletion of bile acids observed upon GvHD induction. The prophylactic use of UDCA has been previously proposed in a study which demonstrated a reduction in aGvHD incidence as well as non-relapse mor- tality with a benefit in overall survival.38 However, other studies failed to confirm this.39,40 Overall, a meta-analysis including four prospective trials and two historical analyses of prophylactic UDCA use in allo-HCT recipients showed a reduction in the levels of hepatic veno-occlusive disease and transplant-related mortality, with no statistically signif- icant difference in the incidence of acute hepatic GvHD or overall survival.41 The impact on intestinal aGvHD inci- dence was not evaluated. Our observations prompted us to search for a mechanism by which bile acids and TUDCA in particular might protect the intestinal epithelium from an alloimmunity-mediated damage. Administration of TUDCA led to reduced expression of antigen presentation- related genes and to reduced expression of MHC class I and II on subpopulations of non-hematopoietic cells in the intestine. It has recently been shown that MHC class II- expressing intestinal epithelial cells are able to present anti-
haematologica | 2021; 106(8)
2143