Page 109 - 2021_07-Haematologica-web
P. 109
Fatigue in CML patients
active=0.85, vigorously active=1.53, very vigorously active=2.58).
Part 2: activity patterns in fatigued and non-fatigued patients
Table 2 shows the characteristics, including hematolog- ic values, of the 138 CML patients who wore the activity monitor and were included in the final analysis. In line with the first part of the study, there was a higher propor-
tion of female patients in the severely fatigued group than in the non-fatigued group (61% vs. 44%, respectively; P=0.039). The employment status did not differ signifi- cantly across the groups (53% vs. 66% employed in the fatigued and non-fatigued groups, respectively; P=0.20). However, total work time was significantly shorter in both severely fatigued male and female patients than in non-fatigued patients (males: 29 ± 12 h/week vs. 38 ± 12 h/week, respectively; P=0.048; females: 15±8 h/week vs.
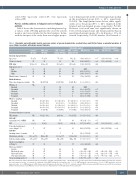
Table 1. Univariable and multivariable logistic regression analysis of general characteristics, medical history, and lifestyle factors as potential predictors of severe fatigue in patients with chronic myeloid leukemia.
Univariable analysis
Multivariable analysis
Controls CML total CML + severe CML + severe (N=110) (N=220) fatigue (N=122) fatigue (N=98)
OR (95% CI)
0.98 (0.96-1.00)
P-value OR (95% CI)
0.07 0.96 (0.93-0.99)
P-value
0.004
0.09
General characteristics
Age,years 56±13 56±13 55±13 58±12
Gender, % female 58 58 66 48
BMI, kg/m2 25.2 ± 3.9 26.2 ± 4.3 26.5 ± 4.6 25.8 ± 4.0
Education level, %
Low 10 14 11 17 REF
0.006 1.76 (0.92-3.34)
2.14 (1.24-3.70)
1.04 (0.98-1.11) 0.24
Mediate 36 42 49 34
High 55 44 40 49
2.38 (1.03-5.50)
0.043
Marital status, % married
Medical history
Time since diagnosis, months
TKI type, % Imatinib
Dasatinib Nilotinib Bosutinib Ponatinib
TKI dose (mg/day) Imatinib
Dasatinib Nilotinib Bosutinib Ponatinib
Treatment duration of current
81 NA
NA
NA
NA
NA
0 (0-0)
83
64 (27-131)
38 27 21
81
61 (27-116)
34 32 19
85
66 (25-140)
44 21 24 7 4
400 (400-400) 100 (85-100) 600 (400-600) 400 (300-400) 30 (19-42)
36 (12-72)
86
2 (2-2)
16
2 1694 ± 528
1.34 (0.59-3.05) 0.49 0.78 (0.38-1.59) 0.49
1.00 (0.99-1.00) 0.46
REF
1.90 (0.96-3.76) 0.07 1.05 (0.51-2.15) 0.90 1.80 (0.65-5.01) 0.26 1.83 (0.50-6.74) 0.36
1.00 (1.00-1.00) 0.77 0.98 (0.95-1.00) 0.13 1.00 (1.00-1.00) 0.45 1.00 (0.99-1.01) 0.58 0.99 (0.89-1.09) 0.77
1.00 (1.00-1.01) 0.48 0.47 (0.21 – 1.0) 0.05
9 10
56
TKI, months
BCR-ABLIS
transcript level, % MMR
CCI
Comedication known to cause fatigue*, % Lifestyle
Smoking status, % smoker
Fluid intake, mL/day
Caffeine intake, mg/day
Alcohol consumption
>1U/day, %
Physical activity#, % Inactive
Moderately active Vigorously active Very vigorously active
Not performing sports, %
7
8 1806 ± 336 ±
17
6 19 28 47 33
400 (400-400) 100 (700-100) 600 (400-600) 400 (300-500) 30 (15-45)
36 (12 – 69)
80
2 (2-3)
27
5 1642 ± 545
400 (400-400) 100 (50-100) 600 (400-600) 400 (300-500) 30 (15-45)
24 (6-60)
75
2 (2-3)
36
7 1601 ± 557
1.67 (1.10-2.53)
2.89 (1.51-5.54)
0.017 1.91 (1.16-3.13) 0.001 3.43 (1.58-7.44)
0.011 0.002
0.19 0.014 <0.001
1760 197
326 ± 13 16
25 30 30 46
175
312 ± 11 22
32 28 17 49
175
344 ± 17 4
16 33 47 42
173
3.40 (0.71-16.4) 0.13
1.00 (1.00-1.00) 0.21 1.00 (1.00-1.00) 0.17
0.61 (0.28-1.34) 0.22 REF
0.39 (0.12 – 1.29)
0.16 (0.05 – 0.52)
0.07 (0.02 – 0.22)
1.35 (0.79 – 2.30) 0.28
0.12 0.43 (0.12 – 1.52) 0.002 0.22 (0.06 – 0.74) <0.001 0.08 (0.02 – 0.26)
Data are presented as mean ± standard deviation, percentages or median (interquartile range). *Benzodiazepines, opioids, beta-blockers, and metformin. #Physical activity was classified into four categories: inactive (<500 metabolic equivalent of task [MET] min/week), moderately active (500-1,499 MET min/week), vigorously active (1,500-2,999 MET min/week), and very vigorously active (>3,000 MET min/week).CML:chronic myeloid leukemia;OR:odds ratio;95% CI:95% confidence interval;BMI:body mass index;REF:reference group;TKI:tyrosine kinase inhibitor; IS: International scale; MMR: major molecular response; CCI: Charlson Comorbidity Index; NA: not applicable.
haematologica | 2021; 106(7)
1879