Page 274 - 2021_06-Haematologica-web
P. 274
Letters to the Editor
ABC
DEF
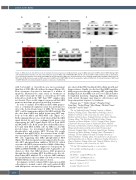
Figure 3. MK2 interacts with AKT to promote multiple myeloma progression. (A) Western blot assay on MK2 expression in ARP1 and OCI-MY5 wild-type (WT) and lentiviral-transfected (OE) cells. (B) Colony formation assay of ARP1 and OCI-MY5 MK2-WT and OE cells treated with or without bortezomib or doxorubicin. (C) Co-immunoprecipitation assay showed that MK2 interacted with AKT in MM cells. (D) Immunofluorescence staining on MK2, AKT and DAPI in ARP1 and OCI- MY5 cells. (E) Western blot assay on pAKT expression in ARP1 and OCI-MY5 MK2-OE cells treated with or without LY292002. (F) Colony formation of ARP1 and OCI-MY5 MK2-OE cells fed by medium in absence or presence of LY292002.
with bortezomib or doxorubicin was more prominent than that of MK2-OE cells without treatment (Figure 3B). Flow cytometric detection for Annexin V, a marker of apoptosis, illustrated the same trend, as treatment on cells with bortezomib (8 nM) or doxorubicin (100 nM) induced less death in the “OE” than “WT” samples (data not shown). These results support our proposal that MK2 promotes myeloma progression and drug resistance.
In order to analyze how MK2 mediates MM progres- sion, a co-immunorrecipitation assay was performed to detect the down-stream target of MK2. We found that AKT could be immunoprecipitated by MK2 antibody. On the other hand, MK2 was pulled down using AKT anti- body in both ARP1 and OCI-MY5 cells (Figure 3C). Further immunofluorescence study showed that the MK2 signal labeled by red color overlapped with green color representing the AKT signal (Figure 3D) in both ARP1 and OCI-MY5 cells. Both assays proved that MK2 direct- ly bound with AKT in MM cells. As MK2 is a Ser/Thr protein kinase, we investigated whether MK2 could phosphorylate and activate AKT. Western blot results confirmed that pAKT(S473), the activated form of AKT, was up-regulated by MK2 overexpression compared to WT cells suggesting that MK2 phosphorylated AKT (Figure 3E). This interpretation was supported by the spe- cific AKT phosphorylation inhibitor, LY490002, which overcame the MK2 activation induced MM cellular drug- resistance and profoundly suppressed clonogenicity in ARP1 and OCI-MY5 OE cells (Figure 3F). A plausible con- clusion is, thus, that MK2 promotes MM progression through directly activating AKT. In addition, we also val- idated that MK2 inhibitor IV, a selective MK2 inhibitor had an inhibititory effect on MM cells both in vitro and in 5TGM1 MM mouse model (data not shown).
In summary, we first evaluated MK2 expression in MM cells relative to normal control cells, and correlated MK2 with MM patient outcomes in relapsed MM patients. We
also showed that MK2 mediated MM cellular growth and drug-resistance. Finally, we disclosed that MK2 regulates MM progression through activating AKT signaling. Our findings indicate that MK2 acts as a novel clinical marker for high-risk myeloma. Targeting MK2 in combination with current therapies may improve effectiveness and long-term patient response to treatment.
Chunyan Gu,1,2,3* Haibo Cheng,4,5* Hongbao Yang,6
Yong Bian,7 Yaohui Wang,8 Yifen Zhang,8 Michael Pisano,2 Gang Hu3# and Ye Yang1,9,10#
1The 3rd Affiliated Hospital, Nanjing University of Chinese Medicine, Nanjing, China; 2Department of Pathology, School of Medicine, University of Iowa, Iowa City, IA, USA; 3School of Medicine and Life Sciences, Nanjing University of Chinese Medicine, Nanjing, China; 4The First Clinical Medical College, Nanjing University of Chinese Medicine, 210023, Nanjing, China; 5Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine Prevention and Treatment of Tumor, Nanjing, China; 6Center for New Drug Safety Evaluation and Research, China Pharmaceutical University, Nanjing, China; 7Laboratory Animal Center, Nanjing University of Chinese Medicine, Nanjing, China; 8Department of Pathology, The First Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China; 9Key Laboratory of Acupuncture and Medicine Research of Ministry of Education, Nanjing University of Chinese Medicine, Nanjing, China and 10Internal Medicine, School of Medicine, University of Iowa, Iowa City, IA, USA.
*YY and GH contributed equally as co-first authors. #CG and HC contributed equally as co-senior authors. Correspondence:
YE YANG - yangye876@sina.com
GANG HU - ghu@njutcm.edu.cn doi:10.3324/haematol.2017.182121 Received: October 7, 2017. Accepted: March 14, 2018.
1776
haematologica | 2021; 106(6)