Page 15 - 2021_06-Haematologica-web
P. 15
Editorials
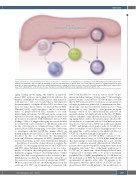
Figure 1. Granulocyte colony-stimulating factor influences the balance of myelopoiesis and lymphopoiesis. Granulocyte colony-stimulating factor (G-CSF) leads to mobi- lization of hematopoietic stem cells (HSC) by disrupting the function and maintenance of specific niche cells. G-CSF enhances expansion of lymphoid (Ly)-biased, short- term HSC in culture and maintains their in vivo repopulating potential. In contrast, G-CSF does not have a direct effect on purified myeloid (My)-biased, long-term HSC. Apart from regulating HSC, G-CSF can also increase myelopoiesis and impair lymphopoiesis by regulating committed progenitor cells.
aging. During mouse aging, the number of myeloid- biased HSC increases more than 10-fold, whereas the number of lymphoid-biased HSC shows only a mild (2- fold) increase.9,10 Xie et al. revealed that G-CSF improves the maintenance of lymphoid-biased HSC in culture, but does not have direct effects on myeloid-biased HSC. Whether G-CSF could contribute to the in vivo mainte- nance of lymphoid-biased HSC remains to be seen. Interestingly, in humans, G-CSF levels in the serum were reported to decrease during aging and this decrease was pronounced in patients with Alzheimer disease.11 The findings of Xie et al. suggest that aging-associated declines in G-CSF level could contribute to the relative reduction in the self-renewal of lymphoid-biased HSC versus myeloid-biased HSC during aging. It would be interesting to investigate whether G-CSF has similar effects on human lymphoid-biased HSC as those on murine HSC described by Xie et al. However, the discrimination between different subtypes of HSC (lymphoid vs. myeloid-biased) has not yet been established in humans.
In addition, it would be of great interest to analyze the influence of other aging-related factors on G-CSF levels and HSC aging. Telomere dysfunction occurs as a conse- quence of telomere shortening and represents one of the hallmarks of aging. Telomere shortening induces cellular senescence and a strong increase in the secretion of pro- inflammatory cytokines by senescent cells - referred to as the senescence-associated secretory phenotype (SASP).12 Of note, senescent cells also show strong increases in the secretion of G-CSF.13 An accumulation of senescent cells
have been described to occur in various tissues of pri- mates, including humans, during aging.14-16 Interestingly, genetic studies on telomerase knockout mice revealed that G-CSF increases in blood serum as a consequence of telomere dysfunction, which led to impairments in lym- phopoiesis and myeloid-skewed hematopoiesis.17 This phenotype is very similar to that present in aging humans, which is also characterized by increases in myeloid relative to lymphoid cells in the blood.18 While studies on human serum showed decreases in G-CSF dur- ing aging, future studies should investigate whether the accumulation of senescent cells in bone marrow tissue may lead to increases in G-CSF levels in the micro-milieu of HSC and lymphoid progenitor cells. If G-CSF does indeed contribute to the reduction in lymphopoiesis dur- ing aging, this could be related to the inhibitory effect of G-CSF on committed lymphoid progenitor cells.2
A direct influence of G-CSF on HSC could also be rele- vant for the clinical usage of G-CSF. It has been shown that macrophage colony-stimulating factor acts directly on HSC to enhance myeloid differentiation, which has positive effects in protecting HSC-transplanted mice from Aspergillus infection.19 Two of the main applications of G-CSF are to ameliorate chemotherapy-induced neu- tropenia and to mobilize HSC to be used for mobilized peripheral blood (MPB) transplantation. G-CSF leads to the mobilization of HSC by disrupting the function and maintenance of specific niche cells.20 It remains to be determined whether direct effects of G-CSF on lym- phoid-biased HSC would influence the mobilization of
haematologica | 2021; 106(6)
1517