Page 142 - 2021_06-Haematologica-web
P. 142
A.B. Arroyo et al.
model of miR-146a deficiency in the hematopoietic line- age caused greater NET formation. However, we previ- ously showed that miR-146a deficiency in the hematopoietic lineage had no effect on atherogenesis after 8 weeks of an atherogenic diet.26 Here, our results suggest that miR-146a deficiency may participate in thrombosis rather than in atherogenesis, through NETosis. This hypothesis is in accord with results pub- lished by Franck et al. demonstrating that NETosis does not alter atherosclerotic plaque formation but rather increases thrombosis in a model of plaque erosion.38 Supporting this hypothesis, previous reports have shown that thrombosis induction increases NET deposition.39,40 Consistently, when arterial thrombosis was induced by FeCl3 injury, we observed an increased generation of NET in the thrombi of miR-146a-/- mice compared to WT mice, with a shorter time to vessel occlusion. The abolition of thrombosis by removal of NET with DNAse I in miR- 146a-/-and WT mice, further supports the essential role of miR-146a in NET production. In this sense, Massberg et al. also found in a murine model of FeCl3 injury that the inhibition of NET components, by infusion of anti-H2A- H2B-DNA antibody, prolonged the occlusion time and generated low stability thrombi in the carotid.39
Next, we created a non-sterile model of inflammation by LPS injection into miR-146a-/- mice. Indeed, miR-146a-/- mice exhibit hyperreactivity to LPS, with an exacerbated inflammatory response, demonstrating miR-146a as a regulator of autoimmunity, myeloproliferation, and can- cer.23 Our results showed that miR-146a deficiency pro- vokes higher rates of NETosis in LPS-challenged mice. Of note, no differences were found in the capacity of phago- cytosis between miR-146a-/- and WT neutrophils in vitro after LPS challenge (data not shown). In addition, miR-146a deficiency promotes a pro-coagulant phenotype with increased levels of thrombin-antithrombin complexes and ROS production and regulates the extent of organ dam- age following infection, as miR-146a-/- mice developed greater lung damage than did WT mice. Although the use of murine models is considered useful for understanding the pathophysiology of sepsis, there are notable differ- ences between these models and sepsis in humans.41 We therefore examined NETosis markers and their relation- ship with thrombosis in CAP patients. Plasma NETosis markers correlated with the incidence of sepsis in accor- dance with previously reported data,42 further supporting the concept that sepsis is a relevant model for investigat- ing the role of NET in humans. Interestingly, we found a correlation between NETosis and thrombotic complica- tions in CAP patients. Our group has previously described that the miR-SNP rs2431697 of MIR146A is associated with an increased risk of cardiovascular events in patients with atrial fibrillation.24 Here, we have verified the role of rs2431697 genotype in cardiovascular events in patients with CAP. Supporting our findings, Xie et al. reported that activation of miR-146a expression decreased markers of myocardial injury in rats treated with LPS.43 In addition, the injection of miR-146a has been described to play a protective role in the cardiac dys- function induced by sepsis in a murine model, with longer survival of the mice.44 Results from our cohort indi- cate that NETosis could be a functional path by which miR-146a levels lead to thrombosis in septic patients. Of note, we found that among patients with higher levels of NETosis markers, carriers of the T allele were 3-fold more
frequent than CC patients. The concept of sepsis and car- diovascular diseases sharing a common pathophysiology has been explored recently.45 Common gene signaling pathways for both sepsis and cardiovascular diseases were investigated and genetic variations in miR-146a tar- gets such as IL6 or IRAK1 were found to be shared in patients with both diseases.45 Although the etiology of sepsis and cardiovascular disease is not identical, the two conditions share the same end-points of inflammation, coagulation, and endothelial activation.46,47 Overall our results demonstrate that miR-146a is involved in throm- bogenesis of two diseases with cardiovascular events, atrial fibrillation and sepsis, a priori in two different mod- els. Accordingly, miR-146a may be yet another regulator of immunothrombosis.
The mechanisms leading to the increased NET forma- tion by miR-146a are still elusive. Interestingly, pro- inflammatory activity of neutrophils has been shown to correlate positively with aging.30 Aged neutrophils are the
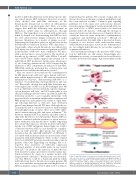
Figure 5. miR-146a plays a relevant role in thrombo-inflammation after sterile and non-sterile stresses. Low miR-146a levels promote an aging phenotype in neutrophils (CD62low CD11bhigh Cxcr4high Tlr4high) which primes these cells. Upon sterile or non-sterile stimuli, primed neutrophils would be more prone to NETosis leading to a thrombo-inflammatory process. MiR-146a: microRNA- 146a; LPS: lipopolysaccharide; CAP: community-acquired pneumonia.
1644
haematologica | 2021; 106(6)