Page 281 - 2021_05-Haematologica-web
P. 281
Letters to the Editor
BCR-mediated CD184 downregulation and lym- phadenopathy in CLL patients.6 In addition, ibrutinib, by inhibiting CD184-mediated signaling through blockade of CXCL12-mediated phosphorylation at CD184 Ser339, could reduce stromal tethering and further enhance CLL cell egress from lymph nodes in vivo.7
CLL cells also showed significantly reduced expression of CD38 and CD49d (n=48/119 CD38+ cases: 874±941 vs. 353±289, P=0.0002 and n=62/119 CD49d+ cases: 1449±1087 vs. 811±790, P<0.00001; respectively), which are associated with a worse prognosis as mediators of leukemic B-cell localization in tissue protective niches, promoting rapid growth and longer cell survival. Furthermore, among cell activation and co-stimulatory molecules, IR treatment induced significant downmodu- lation of CD44 (10999±6845 vs. 8844±5858, P=0.0011), a surface glycoprotein receptor for hyaluronic acid, CD40 (820±493 vs. 445±309, P<0.00001) and CD69 (825±1069 vs. 464±699, P<0.0001). Since the expression of these markers is characteristic of tissue-resident CLL cells,
these results, in line with previous in vitro8 and in vivo9 observations, suggest that ibrutinib may reduce leukemic cell activation, interfering with B-cell survival and prolif- eration. We also recorded a significant downmodulation in the expression of CD43 (3503±2535 vs. 2714±1813, P=0.00005); contrariwise, CD81 expression resulted unchanged after 14 days of treatment (527±661 vs. 423±353, P=0.085). As both antigens are employed in CLL for the flow cytometric detection of minimal resid- ual disease,10 our data suggest that CD43 is not a reliable marker under ibrutinib therapy and that the identification of different fluorimetric panels excluding CD43 should be considered in this context.
In agreement with the changes in MFI levels, ibrutinib treatment also induced significant decreases in the per- centages of CLL cells expressing CD38 (P<0.00001), CD40 (P<0.0001), CD44 (P<0.0001), CD49d (P=0.004), CD62L (P<0.0001), CD69 (P<0.00001) and CD185 (P<0.00001). In contrast, no differences in either MFI or percentage values were observed for CD11a, CD18a,
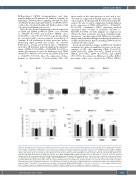
A
B
C
Figure 2. Modulation of the expression of adhesion molecules, chemokine receptors and activation markers in patients with chronic lymphocytic leukemia after 14 days of ibrutinib plus rituximab in vivo treatment, according to cytogenetic aberrations and CD20 expression. (A) Comparison of the changes of expres- sion of adhesion molecules, chemokine receptors and activation markers among the different cytogenetic categories (i.e., tris12, normal karyotype, del13q14, del11q and del17p). Data are presented as the difference between mean fluorescent intensity (MFI) of surface marker expression after 14 days (D14) of ibru- tinib plus rituximab (IR) treatment compared to baseline (D0) (DMFI: D14MFI-D0MFI). Significant differences are indicated as **P<0.01, *P<0.05 (unpaired Student t-test). (B) Correlation between CD20 MFI and chronic lymphocytic leukemia (CLL) median absolute cell counts on D0 and D14 of IR in vivo treatment. CLL patients were subdivided according to a higher (CD20high MFI≥1000) or lower (CD20low MFI<1000) CD20 expression on the basis of the median value of the geometric MFI of the antigen at baseline (*P<0.05, unpaired Student t-test). D0 and D14 CLL median absolute cell counts observed in the totality of samples included in the study are added for comparison. (C) Comparison of changes of expression of adhesion molecules, chemokine receptors and activation markers between CD20high and CD20low CLL patients after 14 days of IR treatment (ΔMFI: D14MFI-D0MFI; ***P<0.001, **P<0.01, *P<0.05, unpaired Student t-test). Data of antigen expression changes observed in the totality of samples analyzed for each antigen are added for comparison.
haematologica | 2021; 106(5)
1501