Page 217 - 2021_05-Haematologica-web
P. 217
2'-O-methoxyethyl splice-switching oligos
A
B
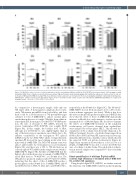
Figure 2. 2'-MOE-SSO induce increase of adult hemglobin production and wild-type b-globin mRNA in IVS2-745/b0 heterozygous sample. (A) Percentage of adult hemoglobin (HbA) from cell lysates as detected by reverse-phase high performance liquid chromatography. (B) Quantitative polymerase chain reaction for only cor- rectly spliced WT b-globin (HBB) messenger RNA. Scale indicates relative expression as normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene and the red-cell specific glycophorin A gene; n=3. S= scramble treated control at same dose. All statistics (ANOVA/Kruskal-Wallis) are compared to scramble control. **P<0.01; ***P<0.001; ****P<0.0001.
les, compared to a heterozygous sample, with only one 745 target allele. A homozygotic sample produces exclu- sively 745 mutant pre-mRNA from both alleles, and thus there could be a 2-fold increase of 745 pre-mRNA target substrate for the 2'-MOE-SSO to induce normal splice switch than in the case of a single 745 allele. In an addition- al specimen (P5), homozygous for the IVS2-745 HBB muta- tion, the ratio of aberrant to WT mRNA detected by reverse transcriptase PCR (RT-PCR) was roughly 50:50 (Figure 5A). In this sample, the baseline level of HbA, although low (6.75±0.22%), was slightly higher than in any of the heterozygous specimens, most likely due to the additive contribution of the endogenous WT spliced mRNA from the two IVS2-745 b+ alleles. As hypothesized, specimen P5 showed the most striking results after treat- ment with 2'-MOE-SSO. The IVS2-745 mutant form was almost undetectable by electrophoresis in all treatments (Figure 5A); and there was a 300- to 700-fold increase in correctly spliced WT mRNA in the samples treated with 2'- MOE-SSO 91 (Figure 5B). All three 2'-MOE-SSO had the highest effect in this homozygous sample. 2'-MOE-SSO 91 raised HbA levels to 79.46±0.94%, at a 50 mM dose. The 100 mM dose produced similar results (77.92±1.04% HbA), indicating that the effect of this 2'-MOE-SSO reaches a protein plateau at the 50 mM dose. 2'-MOE-SSO 92 and 93 raised HbA levels to HbA 71.42±1.22% and 58.48±0.32%,
respectively, at the 50 mM dose (Figure 5C). The 100 mM 2'- MOE-SSO 91 dose in the homozygote led to a 60% reduc- tion in α-heme aggregates (not shown). While at this dose the effect on the protein production plateaued, Q-PCR data show that the effect of these 2'-MOE-SSO dramatically increases at 25 mM dose, and continue to escalate up to the maximum dose of 100 mM (Figure 5B). In order to examine the effects of 2'-MOE-SSO treatment on the α:b-globin stoichiometry, cell lysates obtained from erythroblasts were analyzed by reverse-phase HPLC. This allowed dis- crimination of the α chains from the b-like chains (b, γ, d). Without treatment, the ratio of α:b-like chains ranged from 65:35 in P1 to 55:45, in P4 and P5, respectively, and was sig- nificantly different from a healthy control, which showed the expected 50:50 α:b globin stoichiometry (Figure 6). Single-chain separation showed that at a dose as low as a 25 mM, 2'-MOE-SSO 91 was able to restore the 50:50 bal- ance of α chains to b-like chains. The α:b globin stoichiom- etry in treated samples was comparable to that of healthy control samples.
Direct quantification of wild-type b-globin mRNA confirms high efficiency of treatment with 2'-MOE-SSO 91 on IVS2-745 specimens
Using droplet digital PCR (ddPCR), we further attested the efficiency of 2'-MOE-SSO treatment mediated splicing
haematologica | 2021; 106(5)
1437