Page 157 - 2021_05-Haematologica-web
P. 157
In vivo proplatelet formation
the shrinkage observed after injection of vincristine was dependent on myosin contractility. Again, we previously controlled that microtubules from Myh9-/- mouse platelets and MK were sensitive to vincristine-induced depolymer- ization (Online Supplementary Figure S4; Online Supplementary Figure S5). Strikingly, no PPT shrinkage occurred after vincristine administration in Myh9-/- mice, rather the protrusions continued to elongate so rapidly that they often disappeared from the observation field within 10 minutes (Online Supplementary Video S5; Online Supplementary Figure S6). Increasing vincristine dose to 2 mg/kg did not modify this surprising behavior, as again none of the Myh9-/- protrusions retracted, and elongation continued (Figure 6A-C).
Examination of the bone marrow sections by immuno- fluorescence microscopy showed that Myh9-/- nPPT, like their WT counterparts, do not present a unique micro- tubule bundle (Online Supplementary Figure S7, to compare to Figure 4D-E). Treatment with vincristine did not prevent the observation of long GPIbb-positive protrusions despite the lack of visible microtubules (Figure 6D, arrow). These data indicate that the shrinkage following microtubule depolymerization is dependent on active myosin IIA.
Altogether these data show that in the absence of myosin IIA, microtubules were totally dispensable for nPPT elongation, indicating that elongation can be promot- ed by other driving forces, independent of microtubules.
Stokes’ forces can contribute to native proplatelets elongation
Driving forces contributing to the elongation process could originate from blood flow. This hypothesis was ini- tially supported by the observation that cessation of blood flow induced the relaxation of already preformed three nPTT that extended in the same flow line (Figure 7A; Online Supplementary Video S6), showing that blood flow maintains nPPT under tension.
Flows in sinusoids are complex due to the intricate anas- tomosis of the vasculature (Figure 7B). This was evidenced during the monitoring of platelet movements inside ves- sels of living mice as in some cases areas of inverse flows were observed (Figure 7C; Online Supplementary Video S7). In these areas, nPPT were tossed from one branch of a ves- sel to another, without PTT detachment (Figure 7D; Online Supplementary Video S8). Since they remain attached to the stationary MK in the bone marrow, nPPT are sub- mitted to the fluid force of the flowing blood all along their shafts and buds. Assuming the nPPT end as a sphere, the force can be calculated using the Stokes’ formula F=6πηLV where L is the radius of the sphere, V the veloc- ity of the fluid and η the viscosity. The Stokes’ force applied to the nPPT end was estimated based on the dis- placement of circulating platelets, recorded in vessels where nPPT were extended (V=213 μm/s (range: 24-488) (Figure 7E). The nPPT end having a mean radius of 4 mm
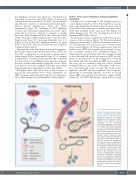
Figure 8. The proposed model depict- ing the cytoskeletal-based differ- ences between in vivo and in vitro proplatelet formation. In cultured proplatelets (cPPT) generated in vitro, initiation and elongation depend essentially on the microtubule cytoskeleton organized as linear bun- dles along the PPT shafts and ending as a coil (1), already prefiguring the marginal band of the future platelets, while F-actin would promote branch- ing. In vivo, the initiation of native pro- platelets (nPPT) formation takes place in the marrow and depends on both actin and microtubule cytoskele- tons (2). During nPPT elongation in the sinusoid circulation, microtubules do not form a unique bundle but are mostly isolated and play a critical role to counteract myosin-based nPPT retraction (3), while drag forces con- tribute to the protrusive forces. The released elongated nPPT fragments are further remodeled in the down- stream microcirculation resulting in the final circulating platelets, possibly through microtubule-based mecha- nisms similar to in vitro mechanisms (4). DMS: demarcation membrane system.
haematologica | 2021; 106(5)
1377