Page 117 - 2021_05-Haematologica-web
P. 117
Vaccine overcomes checkpoint inhibitor limitation
A
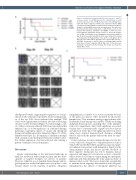
Figure 5. Combination treatment with the fusion vaccine + anti-PD1 or fusion vaccine + anti-TIM3 prevents the establishment of acute myeloid leukemia in vivo. C57BL/6J mice were retro-orbitally inocu- lated with 50x103 syngeneic TIB-49 acute myeloid leukemia (AML) cells that were stably transduced with luciferase/mCherry. Syngeneic dendritic cell (DC)/AML fusion cells were generated. The mice were then treated with either vaccine alone, or with fusion vaccine in com- bination with anti-PD1; anti-TIM3 or anti-RGMb. Control mice were treated with the appropriate isotype control. To assess the progres- sion of AML, (A) bioluminescence imaging was performed starting on day 30 after inoculation and (B) the mice were followed for survival as illustrated in the Kaplan Meier curve (n=5 in each group). At 90 days after the initial tumor challenge, mice treated with vaccine + anti-TIM3; and vaccine + anti-PD-1 were re-challenged with an addi- tional dose of 50x103 syngeneic TIB-49 AML cells. (C) The mice were followed for survival for another 90 days. The results are shown in a Kaplan Meier curve.
B
checkpoint blockade, suggesting the expansion of vaccine- educated cells with tumor specificity. Clone tracking analy- sis of the top TCR clones indicated that multiple TCR clones were significantly modulated (absolute fold-change >2) after vaccine treatment (Figure 7C). Interestingly, a sub- set of these vaccine-modulated TCR clones showed further enhanced up- or down-regulation on combined therapy, indicating a synergistic impact of vaccine and checkpoint therapy in building anti-tumor immunity (Figure 7C, Online Supplementary Figure S2). In summary, combination therapy with vaccination and checkpoint blockade resulted in selec- tive, further expansion of vaccine-educated cells creating a pattern of enhanced clonal dominance.
Discussion
Greater understanding of the mutational landscape in AML has resulted in better prognostication and the devel- opment of targeted therapies for subsets of patients. However, while the use of standard chemotherapy and tar- geted agents has resulted in higher rates of response, a cure remains elusive for the majority of patients. Allogeneic
transplantation is curative for a subset of patients because of the graft-versus-disease effect mediated by alloreactive lymphocytes.2 This treatment strategy suggested that cellu- lar immune-based therapy was capable of fully eradicating malignant hematopoiesis, including that of the primitive stem cell compartment, albeit with significant associated toxicity due to concurrent risks of graft-versus-host disease and infection. There has been significant interest in devel- oping more targeted immune-based therapies utilizing effector cells from the patient without the need for trans- plantation.
A major advance in cancer immunotherapy was the dis- covery of the role of negative co-stimulatory factors such as CTLA-4/B7 and the PD-1/PD-L1 pathways in promoting T- cell exhaustion in patients with malignancy, thereby facili- tating immune escape and disease growth. Blockade of these negative checkpoints has led to dramatic disease regression and improved outcomes in a subset of patients with advanced solid tumors, transforming the therapeutic landscape and demonstrating the truly unique potency of immune effector cells.25 However, despite the susceptibility to immune-based targeting demonstrated with allogeneic transplantation, the efficacy of checkpoint inhibitors has
C
haematologica | 2021; 106(5)
1337