Page 159 - 2021_04-Haematologica-web
P. 159
SYK inhibition for infant ALL
AB
C
D
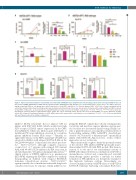
Figure 6. Superior preclinical activity of entospletinib and selumetinib in KMT2A-R acute lymphoblastic leukemia (ALL) patient-derived xenograft (PDX) models. (A) PDX models 142MR (KMT2A-AFF1, NRAS-mutant) and (B) ALL3113 (KMT2A-AFF1, RAS wild-type) were treated with vehicle control chow, 0.05% ENTO chow, 100 mg/kg selumetinib (SEL) via oral gavage twice daily 5 days/week, or both ENTO and SEL for 2 or 4 weeks. Human CD45+ CD19+ ALL cells were quantified by flow cytometry in peripheral blood and end-study murine spleens. Enhanced anti-leukemia efficacy was observed in both models with combined ENTO and SEL treatment versus ENTO or SEL alone, as measured by one-way ANOVA with post Tukey’s post-test for multiple comparisons. *P<0.05, **P<0.01, ****P<0.0001. (C) Ex vivo phosphoflow cytometry analysis of gated human CD19+ CD45+ ALL cells in end-study murine spleens after 2 weeks (ALL142MR) or 4 weeks (ALL3113) of ENTO and/or SEL treatment demonstrate inhibition of pSYK, pERK, and/or pS6 versus vehicle control (gray). ns: not significant, *P<0.05, **P<0.01 by one-way ANOVA with post Tukey’s post-test for multiple comparisons.
inhibitor (FLT3i) lestaurtinib did not improve EFS for infants with KMT2A-R B-ALL (which usually have high FLT3 receptor [CD135] surface expression) in the COG trial AALL0631, which was likely in part attributable to insufficient PD target inhibition observed by correlative plasma inhibitory activity (PIA) assays.50,51 Similarly, no appreciable efficacy of the FLT3i quizartinib (AC220) was observed in children with relapsed KMT2A-R ALL in the TACL2009-004 phase I clinical trial (clinicaltrials.gov identi- fier: NCT011411267), although complete responses occurred in 3 of 7 patients with relapsed FLT3-mutant AML with 94-100% FLT3 inhibition by PIA assay seen in all studied patients.52 Despite promising preclinical data,53,54 clinical activity of DOT1L inhibitors (e.g., pinemetostat [EPZ-5676]) targeting the KMT2A complex was similarly disappointing in children with relapsed KMT2A-R leukemias (clinicaltrials.gov identifier: NCT02141828),55 again potentially due to insufficient achievable drug levels considered necessary for response. Menin inhibitors tar-
geting the KMT2A complex have shown exciting preclin- ical activity and may have superior pharmacologic proper- ties, but have not yet entered clinical testing. Finally, cur- rent or planned trials are assessing the potential activity of 5-azacytidine priming (COG AALL15P1; clinicaltrials.gov identifier: NCT02828358) or blinatumomab56 specifically in infants with KMT2A-R ALL; however, results of these approaches are not yet known.
Our current study sought to define the potential activity of the selective SYK inhibitor ENTO specifically in pre- clinical infant KMT2A-R ALL PDX models. Our demon- stration of in vitro and in vivo anti-leukemia activity of ENTO with enhanced effects in combination with VCR or dexamethasone (critical and commonly-used anti-ALL chemotherapy agents) provides a rationale for further evaluation of SYK inhibition as a therapeutic strategy for this high-risk leukemia subtype. Interestingly, we observed minimal activity of ENTO alone or with VCR in KMT2A-R leukemias harboring concomitant RAS muta-
haematologica | 2021; 106(4)
1075