Page 155 - 2021_04-Haematologica-web
P. 155
SYK inhibition for infant ALL
ABC
DE
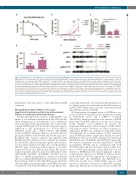
Figure 2. Activity and dose optimization of entospletinib monotherapy in KMT2A-R acute lymphoblastic leukemia (ALL). (A) Viably cryopreserved harvested human KMT2A-R ALL cells from murine PDX spleens (model ALL3103 with KMT2A-MLLT3 fusion) demonstrated dose-dependent inhibition of colony formation in vitro in methylcellulose colony assays after ENTO treatment for 14 days. Samples were plated in triplicate in methylcellulose-based medium and grown in 10% leukocyte- conditioned medium with 25% FBS and 2% BSA. Data are displayed as mean ± SEM. (B) ALL3103 PDX mice were treated with vehicle (control) or ENTO chow at the specified concentrations for 4 weeks. Human CD45+ CD19+ ALL flow cytometric analysis of murine blood at weekly time points and (C) spleens at study endpoint demonstrated significant inhibition of ALL proliferation with ENTO treatment (mean ± SEM). No difference in ALL burden was observed in 0.03% versus 0.07% ENTO- treated animals. (D) Terminal blood was collected from animals after 4 weeks of continuous ENTO chow consumption and evaluated for entospletinib levels. Data from individual animals are plotted as median interquartile range. ns: not significant by t-test. (E) Terminal spleens from individual mice were harvested, viably cry- opreserved, lysed, and evaluated for levels of pSYK, SYK, cMYC, pERK and β-actin by Simple Western. *P<0.05, **P<0.01, ****P<0.0001 as compared to control chow-fed mice by ANOVA with Tukey’s post-test.
dependencies that may relate to their differential ENTO sensitivity.
Entospletinib potently inhibits in vivo acute lymphoblastic leukemia proliferation with enhanced efficacy in combination with chemotherapy
We then investigated the extent to which ENTO could inhibit in vivo leukemia proliferation in ALL PDX models when administered as monotherapy or in combination with vincristine (VCR) chemotherapy. We observed that combined ENTO and VCR treatment resulted in superior inhibition of ALL proliferation in a KMT2A-MLLT3 (ALL3103) model and a KMT2A-MLLT1 (ALL135MR) model (both RAS wild-type) than was observed with sin- gle-agent ENTO or VCR (P<0.001 and P<0.05, respective- ly) (Figure 5A). Superior leukemic cell depletion with ENTO and VCR combination was confirmed by quantita- tive CD19 IHC in harvested murine spleens and bone mar- row (see Online Supplementary Figure S10 for representative ALL3103 data). Conversely, drug treatment of two RAS- mutant KMT2A-R ALL PDX models (Figure 5B) showed marked vincristine-induction reduction of leukemic burden (ALL142MR, P<0.0001; ALL150MD, P<0.001) but no effects of ENTO monotherapy or additional treatment effect of combined ENTO and VCR. Evaluation of an adult RAS wild-type KMT2A-AFF1 ALL PDX model (ALL3113) showed significant treatment effects of ENTO alone and in combination with VCR (P<0.0001 for both) (Figure 5C),
contrasting with effects observed in the RAS-mutant mod- els. Taken together, these data indicate that RAS mutations in KMT2A-R subtypes may overcome or prevent potential anti-leukemia activity of ENTO.
We then explored treatment effects of ENTO in a con- trol non-KMT2A-R ALL PDX model with t(1;19) resulting in TCF3-PBX1 fusion and a KRASG12D mutation (ALL132GD), which we expected to be sensitive to ENTO given typical pre-BCR expression on this more mature B-ALL subtype42,43 and confirmed by positive FC immunoglobulin m-heavy chain staining on AALL132GD cells (data not shown). However, we saw no response to single-agent ENTO or in combination with VCR, further substantiating the potential impact of RAS mutations upon ENTO insensitivity (Figure 5D). Finally, we tested ENTO and VCR in two RAS wild-type non-KMT2A-R ALL PDX models (ALL185GD and ALL83GD) (Figure 5E). We observed sensitivity of model ALL185GD to ENTO monotherapy (P<0.05) and in combination with VCR (P<0.0001), although the latter effects did not differ from those of VCR monotherapy. Model ALL83GD was not sensitive to ENTO alone, but showed significant combina- torial treatment efficacy versus ENTO or VCR monothera- py (P<0.0001 and P<0.05, respectively). Interestingly, we discovered that the ALL185GD and ALL83GD non- KMT2A-R models have P2RY8-CRLF2 fusions with expected constitutive activation of JAK/STAT signaling (Figure 4B). Our group recently reported an essential role
haematologica | 2021; 106(4)
1071