Page 277 - 2021_02-Haematologica-web
P. 277
Letters to the Editor
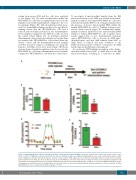
sorting from mouse BM and the cells were analyzed ex vivo (Figure 3A). The mitochondrial mass within the OCI-AML3-luc cells was not significantly reduced in the animals treated with daratumumab compared to the con- trol animals (Figure 3B). Mitochondrial potential meas- ured by tetramethylrhodamine, methyl ester, perchlorate staining shows that the OCI-AML3-luc cells had a reduced mitochondrial potential in the daratumumab- treated animals compared to the NSG mice who received the control vehicle (Figure 3C). To determine whether daratumumab altered mitochondrial-based metabolism, we analyzed the OCI-AML3-luc cells isolated from the vehicle control and the daratumumab-treated animals and then measured oxygen consumption rate using the Seahorse Cell Mito Stress Test assay. Figure 3D shows decreased mitochondrial respiration was observed in the OCI-AML3-luc cells from daratumumab-treated animals compared to OCI-AML3-luc cells from the control mice.
To investigate if mitochondrial transfer from the BM microenvironment to the AML was altered with daratu- mumab treatment, the human OCI-AML3-luc cells were isolated from murine BM by cell sorting and analyzed for the presence of mouse mitochondrial DNA within the human OCI-AML3-luc cells by TaqMan real-time poly- merase chain reaction. Figure 3E confirms that daratu- mumab treatment inhibited mouse mitochondrial DNA transfer to human OCI-AML3-luc cells. Together, these results suggest that, in vivo, daratumumab treatment causes OCI-AML3-luc cells to decrease in AML mito- chondrial mass, and that AML transfer from MSC to AML blasts is reduced and may contribute to the inhibitory functional bio-energetic consequence in AML metabolism in the BM microenvironment.
In conclusion, CD38 inhibition results in reduced mito- chondrial transfer from MSC to AML blasts in the BM microenvironment, resulting in a reduction in AML-
A
B
C
D
E
Figure 3. Daratumumab (Dara) alters the metabolic function of acute myeloid leukemia (AML) cells in vivo. (A) Schematic of the in vivo model for these exper- iments. 0.5x106 OCI-AML3-luc cells were injected into the tail vein of NSG mice. Mice were imaged using bioluminescence at day 9 following injection to confirm tumor engraftment, and then split into two groups. Group 1 received vehicle (PBS) and group 2 received Dara (5 mg/kg) treatment on days 9 and 16 by intra- peritoneal (IP) injection. Mice were then sacrificed at day 21 and OCI-AML3-luc cells were isolated by cell sorting from mouse bone marrow and analyzed for (B) mitochondrial content by MitoTracker green staining. (C) Mitochondrial potential by tetramethylrhodamine, methyl ester, perchlorate (TMRM) staining. (D) Oxygen consumption rate (OCR) by Seahorse Cell Mito Stress Test kit. (E) Presence of mouse mitochondrial DNA by real-time polymerase chain reaction. We used the Mann-Whitney U test to compare results between groups. *P<0.05.
haematologica | 2021; 106(2)
591