Page 160 - 2020_11-Haematologica-web
P. 160
2648
Letters to the Editor
C
AB
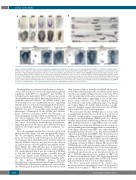
Figure 2. Chicken NPAS4L gene expression during hemangioblast specification. (A) Whole-mount in situ hybridization (WISH) of NPAS4L from HH3 to HH10. Fertilized hen's eggs were purchased from Takamoriryo in Aso (Kumamoto, Japan). (Top) White background for expression visualization; (bottom) dark back- ground for stage visualization. Black lines indicate section levels shown in (B). (B) Section of embryos in (A). NPAS4L-expressing cells indicated by red arrows. Germ layers marked by black arrowheads (ectoderm and endoderm) and brackets (mesoderm). (C) Chicken NPAS4L is expressed starting from HH3+, earlier than SCL/TAL1. Embryos were fixed and processed to the pre-hybridization step (left panels) and were cut into left (stained for NPAS4L) and right (stained for SCL/TAL1 and Chordin together) halves. Stained half embryos were then photographed together (middle panels: white background showing both halves; right panels: dark background showing both halves). Chordin expression was used to mark precise embryo stages. At HH3+ (top row) and HH4 (middle row), NPAS4L is expressed and SCL/TAL1 is not expressed. At HH5 (bottom row), both NPAS4L and SCL/TAL1 are expressed.
Hemangioblast specification from their mesoderm pre- cursors was reported to involve divergent transcriptional regulation, with ETV2 in mammals3,20 and NPAS4L in zebrafish4 as the main driver. ETV2 ortholog is present in the zebrafish genome, but its function was reported to be under the control of NPAS4L.4,6 No NPAS4L ortholog has been identified in any mammalian species, suggesting that this gene is not involved in hemangioblast specifica- tion in mammals. Mammalian NPAS4, a homolog of NPAS4L, was able to rescue fish cloche (npas4l) mutant phenotypes.4 Duplication of the NPAS4 and NPAS4L genes, however, took place before the divergence of Actinopterygians (ray-finned fish, including the teleosts) and Sarcopterygians (lobe-finned fish, including the tetrapods) and NPAS4 has not been associated so far with any aspect of vertebrate hematopoietic development, suggesting that these two genes have different biological functions involving separate molecular regulatory net- works.
Since the mammals and birds are closely related both phylogenetically (Figure 1A) and ontogenetically (Figure 1B), we investigated whether avian NPAS4 and ETV2 genes are involved in early hematopoietic and vascular development. Molecular phylogenetic analysis indicated that an NPAS4L ortholog was present in the chicken (G. gallus) genome (in both galGal5 and galGal6 assem- blies) (Figure 1C). Although this gene is annotated as NPAS4 in the current assembly, syntenic analysis (Figure 1C) clearly indicated that it was the ortholog of NPAS4L in fish and other vertebrate groups (viewable through search term “npas4” in the NCBI genome data browser https://www.ncbi.nlm.nih.gov/genome/gdv/?org=gallus-gallus or the chicken FANTOM dataset browser http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=b1zZI1gUF Z6mHX6-4Gvxr). Phylogenetic analyses also showed that the NPAS4L gene is present in all other bird species with
their genomes fully or partially assembled and in non- avian reptiles with their genomes assembled (Anolis lizard shown as an example in Figure 1C). In contrast, the ETV2 ortholog is missing in the entire avian lineage, and also in crocodiles and turtles, suggesting a loss of this gene before avian evolution. The ETV2 ortholog, however, was found in some of the reptilian lineages (e.g., lizards and snakes) (Figure 1C and D). Taken together, our phy- logenetic analyses suggest that birds have the NPAS4L, but not the ETV2, gene in their genomes.
We next asked whether NPAS4L plays a role in early hemangioblast specification in chick as was shown in zebrafish. For this purpose, we generated an RNA whole- mount in situ hybridization (WISH) probe for chicken NPAS4L and performed WISH using embryos from stage HH3 (early gastrulation) to stage HH12 (onset of circula- tion). Expression of chicken NPAS4L was detected in ter- ritories marking nascent hemangioblasts in ventral meso- derm (Figure 2A) from stage HH3+, the earliest among all hemangioblast-specific genes (e.g., SCL/TAL1 and LMO2 expression starts from stage HH4+). This observation was confirmed by WISH using left-right bisected embryos, with the left half stained for NPAS4L and the right half stained for SCL/TAL1 and Chordin (Figure 2C). Paraffin- sectioning of stained embryos (Figure 2B) showed that NPAS4L-positive cells are located in a subset of the meso- derm germ layer that will give rise to blood and endothe- lial cell lineages (red arrows; germ layers marked by arrowheads and brackets), as we had previously report- ed.10,21 NPAS4L expression levels peaked at HH7 and declined soon afterwards (Figure 2A), suggesting that this gene is specifically and transiently involved in heman- gioblast formation, but not in their differentiation.
We have previously generated the chicken pro- moterome database, spanning the entire 21-day period of embryonic development.22 When we searched this data-
haematologica | 2020; 105(11)