Page 111 - Haematologica Atlas of Hematologic Cytology
P. 111
CHAPTER 13 - Acute myeloid leukemia and related precursor neoplasms
Chapter 13. ACUTE MYELOID LEUKEMIA AND RELATED PRECURSOR NEOPLASMS
Acute myeloid leukemia (AML) is a clonal, neoplastic disorder of the bone marrow characterized by defective maturation of one or more myeloid hematopoietic cell lines, with accumulation of blasts in the bone marrow and in the peripheral blood. The neoplastic clone may belong to the granulocytic, monocytic, erythroid or me- gakaryocytic lineage, although frequently there is simultaneous involvement of all these cell lineages. The neo- plastic transformation generally occurs in the stem cells, at different levels of differentiation depending on the subtype of leukemia (Table 1).
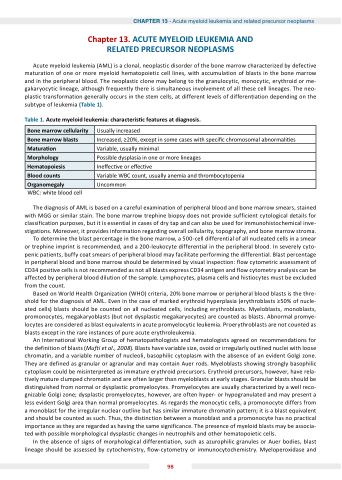
Table 1. cute myeloid leu emia characteris c features at diagnosis.
WBC: white blood cell
The diagnosis of AML is based on a careful examination of peripheral blood and bone marrow smears, stained with MGG or similar stain. The bone marrow trephine biopsy does not provide sufficient cytological details for classification purposes, but it is essential in cases of dry tap and can also be used for immunohistochemical inve- stigations. Moreover, it provides information regarding overall cellularity, topography, and bone marrow stroma.
To determine the blast percentage in the bone marrow, a 500-cell differential of all nucleated cells in a smear or trephine imprint is recommended, and a 200-leukocyte differential in the peripheral blood. In severely cyto- penic patients, buffy coat smears of peripheral blood may facilitate performing the differential. Blast percentage in peripheral blood and bone marrow should be determined by visual inspection: flow cytometric assessment of CD34 positive cells is not recommended as not all blasts express CD34 antigen and flow cytometry analysis can be affected by peripheral blood dilution of the sample. Lymphocytes, plasma cells and histiocytes must be excluded from the count.
Based on World Health Organization (WHO) criteria, 20% bone marrow or peripheral blood blasts is the thre- shold for the diagnosis of AML. Even in the case of marked erythroid hyperplasia (erythroblasts ≥50% of nucle- ated cells) blasts should be counted on all nucleated cells, including erythroblasts. Myeloblasts, monoblasts, promonocytes, megakaryoblasts (but not dysplastic megakaryocytes) are counted as blasts. Abnormal promye- locytes are considered as blast equivalents in acute promyelocytic leukemia. Proerythroblasts are not counted as blasts except in the rare instances of pure acute erythroleukemia.
An International Working Group of hematopathologists and hematologists agreed on recommendations for the definition of blasts (Mufti et al., 2008). Blasts have variable size, ovoid or irregularly outlined nuclei with loose chromatin, and a variable number of nucleoli, basophilic cytoplasm with the absence of an evident Golgi zone. They are defined as granular or agranular and may contain Auer rods. Myeloblasts showing strongly basophilic cytoplasm could be misinterpreted as immature erythroid precursors. Erythroid precursors, however, have rela- tively mature clumped chromatin and are often larger than myeloblasts at early stages. Granular blasts should be distinguished from normal or dysplastic promyelocytes. Promyelocytes are usually characterized by a well reco- gnizable Golgi zone; dysplastic promyelocytes, however, are often hyper- or hypogranulated and may present a less evident Golgi area than normal promyelocytes. As regards the monocytic cells, a promonocyte differs from a monoblast for the irregular nuclear outline but has similar immature chromatin pattern; it is a blast equivalent and should be counted as such. Thus, the distinction between a monoblast and a promonocyte has no practical importance as they are regarded as having the same significance. The presence of myeloid blasts may be associa- ted with possible morphological dysplastic changes in neutrophils and other hematopoietic cells.
In the absence of signs of morphological differentiation, such as azurophilic granules or Auer bodies, blast lineage should be assessed by cytochemistry, flow-cytometry or immunocytochemistry. Myeloperoxidase and
one marrow cellularity
Usually increased
one marrow blasts
Increased, ≥20%, except in some cases with speci c chromosomal abnormali es
Matura on
Variable, usually minimal
Morphology
Possible dysplasia in one or more lineages
Hematopoiesis
Ine ec ve or e ec ve
lood counts
Variable WBC count, usually anemia and thrombocytopenia
rganomegaly
Uncommon
98