Page 231 - 2019_03-Haematologica-web
P. 231
Somatic mosaicism at different stages of ontogenesis
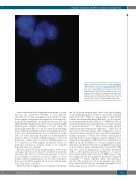
Figure 4. Dual-color fluorescence in situ hybridization signal patterns of selected cell nuclei obtained with PAC clones that encompass the RHD/RHCE (FITC, green) and, as a control, AF1q gene sequences (Cy3, red). Representative results of fixed peripheral blood cells of proposita B are shown: all segmented (top) and round (bottom) nuclei contained two signals each and, thus, two RH gene loci. An identical pattern was seen in proposita A (not shown). Original magnification x1000.
results with mixed-field agglutination patterns is essen- tial for safe transfusion therapy of such patients. Unequivocal blood group typing is a prerequisite for trans- fusion support and prenatal investigations evaluating feto- maternal incompatibility. At many institutions, not only ABO and RhD typing is performed, but also further highly immunogenic antigens including c, K and others are increasingly taken into account for transfusion matching. Such extended matching strategy was markedly shown to reduce the alloimmunization rate of transfusion recipi- ents,28 an effect especially desirable for multi-transfused patient cohorts or women of childbearing age.28-32 For both propositae, neither anti-c nor anti-C alloimmunization is to be expected, as both antigens are present. Hence, no particular transfusion strategy seems to be required regarding these two antigens.
Of note, mixed blood group phenotypes often escape serological detection but may be unveiled by molecular screening. The latter is of particular relevance for blood donor testing: it could have avoided a number of docu- mented anti-D immunizations by red cell concentrates from serologically D-negative blood donors with an unde- tected D-positive cell subset.18
Apart from these implications for transfusion medicine,
the blood group anomaly may only be the first evidence of an underlying genetic alteration of possibly extended clinical relevance. While it is increasingly recognized that somatic mosaicism including LOH may not be uncom- mon in apparently healthy subjects,33,34 LOH-based blood group discrepancy may well represent a surrogate marker of myeloid diseases.4,11,13,35,36 Apart from acute myeloid leukemia and myelodysplastic syndrome,37,38 allelic loss on 1p was also detected in many other malignancies, such as colorectal cancer, neuroblastoma, lung cancer and hepato- cellular carcinoma.39-42 Hence, this chromosomal region is probably home to tumor suppressor genes. It may be con- cluded that, depending on individual tissue distribution of LOH on 1p, the potential loss of tumor suppressor gene function could increase the risk for malignant transforma- tion in affected organs. Alternatively, copy-neutral LOH may also result in duplication of oncogenic mutations with a subsequently increased likelihood of cancer.35 Recent data indicate that detection of LOH may not only have diagnostic but also prognostic potential for myeloid neoplasms.6,37,43 Taken together, when encountering a patient with spontaneous blood group phenotype split- ting, clinical and laboratory screening investigations for hematologic disease should be considered.
haematologica | 2019; 104(3)
637