Page 164 - Haematologica-April 2018
P. 164
D. Belloni et al.
A
BC
D
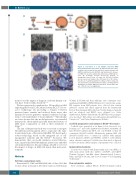
Figure 1. Generation of a 3D multiple myeloma (MM) microenvironment in bioreactor. (A) Experimental procedure: scaffold is pre-seeded in vitro with bone marrow stromal cells (BMSC)/endothelial cells (HUVEC) and transferred to bioreac- tor. MM cells are then added and cultured (see Methods sec- tion). (B) Scanning electron microscopy analysis of Spongostan (bar=20 mm). (C) Input (t0) (200x103/scaffold) and recovered cell number after 18 hours (h) of 3D culture. Results are mean±Standard Error of Mean of three independ- ent experiments. (D) Immunohistochemistry showing uniform distribution of CD138+ MM cells and CD73+ stroma. Bar=100 mm. *P≤0.05. n.s.: not significant.
708
ment to test the impact of drugs in a relevant human con- text have been recently described.10-13
We have previously contributed to 3D models for MM exploiting the Rotary Cell Culture System (RCCSTM) biore- actor technology. By providing a balance between increased mass transfer and reduced shear stress, this dynamic bioreactor generates optimal conditions for long- term ex vivo maintenance of tissue explants.14-16 Specifically, we have shown that the model preserves, for extended time periods, the morphological and functional features of MM tissue components as well as their sensitivity to drugs.16
The aim of the present study was to recreate a surrogate 3D MM microenvironment able to reproduce the func- tional interactions of the native MM BM. We developed a robust technology, based on the integrated use of cell- repopulated scaffolds and the RCCSTM bioreactor. We demonstrate that our model simulates crucial MM fea- tures, in particular BM-MM dynamic interactions and MM survival/proliferation, thus providing a reliable tool to test the impact of drugs on MM cells inside their microenvi- ronment.
Methods
Cell lines and primary cells
Human MM1.S, U266 and RPMI.8226 MM cell lines, HS-5 BM stromal cell line and murine L-fibroblasts transfected with human
VCAM1 (L-VCAM) and their wild-type (wt) counterpart were maintained in DMEM or RPMI 1640 plus 10% fetal bovine serum. BM aspirates from MM patients were collected after written informed consent and ethical approval from the Institutional Review Board; primary MM cells from 7 newly diagnosed patients and one relapsed, and BMSC were obtained (see Online Supplementary Methods). Endothelial cells (HUVEC) were propagat- ed as described.17 Osteoblasts were differentiated from BMSC (see Castrén et al.18 and Online Supplementary Methods).
Scaffold preparation and culture in RCCSTM bioreactor
Scaffolds were generated as in Figure 1A in bioreactor (see Ferrarini et al.16 and Online Supplementary Methods), with MM cell lines (500x103/scaffold) and HS-5 cells or L-VCAM1 or their wt counterpart (200x103/scaffold). Alternatively, primary MM cells (200x103/scaffold) were co-cultured in scaffolds with primary pooled allogeneic BMSC and HUVEC (100x103 each/scaffold), unless otherwise indicated. Scaffolds were then fixed or digested for flow cytometric (FACS) analysis. Supernatants were collected.
Immunohistochemistry
Sections were stained with hematoxylin and eosin (H&E), or with monoclonal antibodies (mAbs): anti-Ki67, anti-CD138 (Ventana Systems); anti-light chains (Immunological Sciences, Italy); anti-CD73 (abcam), anti-cleaved caspase-3 (Cell Signaling Technology).16
Flow-cytometric analysis
Flow cytometric analysis (FACS) (FC500, Beckman Coulter)
haematologica | 2018; 103(4)