Page 156 - Haematologica-April 2018
P. 156
A.-S. Michallet et al.
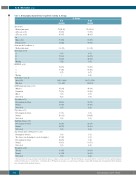
Table 1. Demographic characteristics for patients receiving 1L therapy.
1L therapy
Age (years)
Median (min, max) ≥65 years, n (%) ≥75 years, n (%)
Sex
Male, n (%)
Female, n (%)
Active medical conditions, n
Median (min, max)
Binet stage, n (%)
R-B (N=121)
72 (41, 86) 86 (71) 45 (37)
70 (58)
51 (42) 3 (0, 12)
R-Clb (N=120)
72 (38, 91) 90 (75) 44 (37)
80 (67)
40 (33) 3 (0, 18)
A 6(5) 8(7)
B 73 (60)
C 37 (31)
Missing 5 (4)
ECOG PS, n (%)
0 62 (51)
1 50 (41)
2 9(7) 8(7)
66 (55) 43 (36) 3 (3)
Missing
Body surface area, m2
Mean (SD)
Min, max
IGVH mutational status, n (%)
Mutated Unmutated Othera
Not tested
11q status, n (%)
Heterozygous deletion Normal
Not tested
17p status, n (%) Heterozygous deletion Normal
Not tested
11q/17p deletion, n (%)
Heterozygous deletion Normal
Not tested
13q deletion (S25 or S319 probe)b, n (%) Homozygous deletion
Two clones (one homozygote, one heterozygote) Heterozygous deletion
Normal
Not tested
Trisomy 12, n (%)
Trisomy Normal Not tested
0
1.811 (0.2382)
1.30, 2.48
41 (34) 73 (60) 3 (3) 4 (3)
24 (20) 96 (79) 1 (1)
10 (8) 110 (91) 1 (1)
32 (26) 88 (73) 1 (1)
3 (3) 15 (12) 42 (35) 61 (50) 1 (1)
30 (25) 90 (74) 1 (1)
2 (2)
1.807 (0.1706)
1.41, 2.29
46 (38) 59 (49) 8 (7) 7 (6)
19 (16) 99 (83) 2 (2)
3 (3) 114 (95) 3 (3)
22 (18) 96 (80) 2 (2)
1 (1) 6 (5) 5 (4) 60 (50) 2 (2)
19 (16) 99 (83) 2 (2)
59 (49) 51 (43)
700
aOther includes polyclonal and oligoclonal. bDeletion status according to at least one probe (NB, in the R-B group, one patient with two clones by S319 probe and heterozygous deletion by S25 probe is counted twice). 1L: first-line; ECOG PS: Eastern Cooperative Oncology Group performance status; R-B: rituximab plus bendamustine; R-Clb: rituximab plus chlorambucil; SD: standard deviation.
haematologica | 2018; 103(4)