Page 31 - Haematologica3
P. 31
EVs in the hematopoietic microenvironment
tor-expanded wild-type HSCs ameliorated the inflamma- tory state. While Rab27DKO mice showed no response to lipopolysaccharide, response could be restored following exposure to wild-type hematopoietic EVs, but not EVs harvested from miR-155-/- cells, indicating that vesicle traf- ficking of miR-155 regulates the innate immune response.88
Perspective and open questions
HSCs are competitively displaced from the hematopoi- etic niche in several types of cancer. Yet, the successive loss of HSCs from the BM is not explained by mere physical displacement, but rather occurs even at a disproportionate- ly low tumor burden or with extramedullary tumor loca- tion. The underlying mechanisms of how cancer cells are able to disrupt the hematopoietic niche still remain unclear.8,89,90 Cellular competition was historically charac- terized in Drosophila as a non-cell autonomous mecha- nism involving p53 as a rheostat, whereby healthy (low p53) cells can competitively eliminate damaged and func-
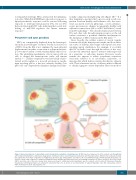
tionally compromised neighboring cells (Figure 4B).91,92 It is thus tempting to speculate that a process such as cell com- petition between healthy and leukemic cells, the senes- cence-associated secretory phenotype, or even submicro- scopic pre-cancerous changes in apparently healthy cells adjacent to tumor tissue (the so called “field effects”), result from EV trafficking.92,93 The described interaction between EVs and other cells through integrin receptor on the cell surface constitutes a potential candidate mechanism for the disruption of HSC retention in the BM niche.3,13,94
More broadly, the cellular context of vesicle transfer between cells in the hematopoietic niche, and how differ- ent routes of delivery affect target cell response are areas requiring urgent clarification. For example, it is evident that EV crosstalk occurs through the release of free vesi- cles into the interstitial space to interact with target cells in a paracrine or endocrine manner. However, vesicle transfer also appears to utilize cytoplasmic extensions (variously referred to as cytonemes, nanotubes, or invadopodia) which deliver contents directly into adjacent cells. These alternative modes of delivery make it difficult to cleanly segregate contact-dependent effects from those
Figure 4. Unresolved aspects of extracellular vesicle biology in the regulation of hematopoiesis. (A) EVs have been proposed to enter recipient cells through lipid raft-mediated internalization, endocytosis, phagocytosis, membrane fusion, caveolin-mediated endocytosis and macropinocytosis. (B) Exosome-mediated crosstalk may explain the intercellular competition of neighboring cells where the “winner” HSPC outcompetes the less fit HSPC through a P53-dependent mechanism. (C) Vesicles contain cargo comprised of uniquely packaged proteins, miRNAs and RNAs which serve as promising biomarkers for disease detection. (D) Vesicles from HSPCs and other cells of the bone marrow niche have been shown to exhibit preferential targeting to specific recipient cells for entry. (E) Cytonemes (filopodia, invadopodia, tunneling nanotubes) are cytoplasmic extensions that serve as modes of exosomal transfer to adjacent bystander cells. EVs: extracellular vesicles; HSPC: hematopoietic stem and progenitor cell.
haematologica | 2018; 103(3)
391