Page 23 - Haematologica3
P. 23
EVs in the hematopoietic microenvironment
stream (Figure 1B).10,12 However, given their complex cargo and poorly understood selectivity for cellular uptake, many phenotypic outcomes are not easily explained by conventional models of cell-cell crosstalk. The conse- quences of simultaneously transferring an unknown num- ber of non-randomly assembled proteins and RNA to another cell defy the clear predictions that apply to more conventional receptor-ligand signaling. However, while an understanding of the molecular basis for EV crosstalk is in its infancy, the key principles of how EVs shape tissue function are beginning to emerge.12 Several groups have recently demonstrated that EVs contribute to the compart- mental regulation of hematopoiesis in the BM.13,14 In this review, we present current evidence for the role of EVs in both homeostatic and pathogenic hematopoietic niches with emphasis on regulatory mechanisms, experimental outcomes and the critical open questions in the field.
Extracellular vesicles
EVs are membrane-enclosed structures of varying size (30-10,000 nm) released from cells to mediate both local and distant intercellular communication. Platelet-derived vesicles were first identified by electron microscopy over 50 years ago,15 yet the full spectrum of subtypes and activ- ities of EVs have only become a major focus of interest in recent years. In the early 1980s, it was reported that sheep reticulocytes selectively release transferrin receptor within EVs during programmed enucleation of the maturing red cell and were generally considered to simply reflect the export of cellular waste.16 Recent studies of EVs in the BM have shown that these vesicles serve to regulate hematopoiesis, participate in immune cell activation, and act as mediators of hemostatic functions.11,17,18 Hematologic malignancies such as leukemia, multiple myeloma or viral infections can coopt EV trafficking
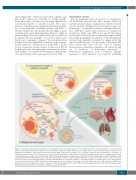
Figure 1. Schematic representation of biogenesis of extracellular vesicles and unique aspects of their trafficking. (A) The conventional model of cellular crosstalk involves receptor-ligand interactions between secreted chemokines, cytokines and growth factors and cellular surface receptors. (B) EV-mediated crosstalk occurs through the trafficking of vesicle-associated protein, lipid and RNA components to proximal cells or to distal organs via the bloodstream in a “paracrine” or “endocrine” manner, respectively. (C) Exosomes are formed from the maturation of early endosomes into Rab7-containing late endosomes leading to the generation of intraluminal vesicles via the action of tetraspanin and ESCRT proteins which sort the endosomal constituents into distinct multivesicular bodies. Through the action of Rab27 and VPS33b, multivesicular bodies evade lysosome degradation and fuse with the plasma membrane to release 30-125 nm exosomes. Cells also release 50-1000 nm microvesicles that form through calcium-mediated budding of the plasma membrane, and during programed cell death, large (>1000 nm) apoptotic bodies. ApB: apoptotic bodies ESCRT: endosomal-sorting complex required for transport; GF: growth factors; ILV: intraluminal vesicle; MV: microvesicle; MVB: multi- vesiclular bodies; mTOR: mammalian target of rapamycin; PI3K; phosphatidylinositol-3 kinase; TGF-β: transforming growth factor beta; TGN: trans-Golgi network; TSPAN: tetraspanin; VPS33B: vacuolar protein sorting-associated protein 33B.
haematologica | 2018; 103(3)
383