Page 21 - 2020_08-Haematologica-web
P. 21
Editorials
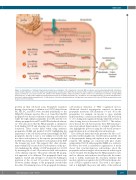
Figure 1. Carboxylation of vitamin K-dependent proteins by γ-carboxylase. The endoplasmic reticulum (ER) membrane-associated gamma-glutamyl carboxylase (GGCX) modifies glutamic acid (red) to gamma-carboxy-glutamatic acid (Gla, blue) within the Gla domain. GGCX recognizes and binds the substrate via the propeptide region (red helix) in a processive fashion. The affinity of the GGCX-propeptide complex determines relative efficiency of carboxylation as follows: 1) high affinity propeptides (Kd ~1 nM) result in significant uncarboxylated protein; 2) low affinity propeptides (Kd ~20 nM) are associated with moderate to normal carboxylated pro- tein; and 3) optimal affinity propeptides (Kd ~5 nM) produce efficiently carboxylated protein. Glu: glutamic acid residues; FIX: factor IX; PTM: post-translational mod- ification; C-term: C-terminus.
proteins in their cell-based assay. Propeptide sequences having a broad range of affinities for GGCX derived from FX, FIX, PC, and BPG were attached individually to the FIXgla-PC chimeric reporter. Hao et al. found that the FIX propeptide was the most efficient at directing carboxylation while the high affinity propeptide from FX and the low affinity propeptides from PC and BGP had reduced efficien- cy.14 The data show that the FIX propeptide is optimal for both binding GGCX and releasing once the protein is car- boxylated. These results differ when using synthetic propeptides, FLEEL and purified GGCX,9 highlighting the importance of the cell-based system. Interestingly, the BGP propeptide, known to have a low affinity for GGCX, did not direct carboxylation of the reporter protein harboring the FIX Gla domain, but did direct carboxylation if the BGP Gla domain was used. This suggests that other determi- nants within BGP are needed for carboxylation of this pro- tein. Enhancing the affinity of BGP propeptide for GGCX by mutating the -6 and -10 position rescued carboxylation of the chimeric reporter. The picture with the FX propep- tide appeared to be different. This propeptide binds very tightly to GGCX and attempts to weaken the binding by mutation at the -6 and -10 position were unsuccessful. However, further changes to the propeptide revealed that the entire N-terminal portion of the propeptide determines
carboxylation efficiency of VKD coagulation factors. Additional detailed investigation centered on known propeptide mutations. FIX mutations (-9 and -10 in the propeptide), for example, are known to cause warfarin hypersensitivity; a situation in which active FIX levels drop to <1% during anticoagulation therapy while the activity of other clotting factors is decreased to 30-40%.10 The authors show that, in the cell-based system, these FIX mutant pro- teins were indeed hypersensitive to warfarin. Again, these data highlight the power of using the cell-based system to gain information about clinically relevant mutations.
The cell-based functional study presented by Hao et al.14 provides further insights into GGCX function and the role of the propeptide during carboxylation in its natural envi- ronment. The findings are consistent with prior studies using purified GGCX and propeptide/FLEEL as a substrate. However, the work is nonetheless significant as it nicely shows that structure/function relationships about the propeptide and new insights about mutations in this region can be obtained. The finding that the FIX propeptide is opti- mal for efficient carboxylation should provide the frame- work to further understand the structural elements that mediate substrate recognition by GGCX and in the produc- tion of VKD coagulation factors. The work is also impor- tant as it highlights the power and utility of the cell-based
haematologica | 2020; 105(8)
1997