Page 119 - 2020_07-Haematologica-web
P. 119
Transforming activities of the NUP98-KMT2A fusion
ABC
DEF
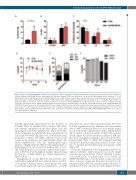
Figure 2. Expression of iNUP98-KMT2A results in LSK cell expansion with a competitive repopulation advantage. (A) Lineage negative (Lin-) bone marrow (BM) cells from pre-leukemic iNUP98-KMT2A mice and wildtype (WT) littermate controls (CTRL) were stained for c-Kit and Sca-1 and analyzed by flow cytometry. *P<0.05, unpaired t-test. (B) Lin- BM from pre-leukemic iNUP98-KMT2A mice and WT littermate controls were stained for markers of long-term hematopoietic stem cells (LT- HSC) (LSK, CD150+, CD48-) and multipotent progenitors (MPP) (LSK, CD34+, CD48+, CD150-) and analyzed by flow cytometry. The percentages of LSK identified as LT-HSC and MPP are shown. (C) Cell cycle analysis of LSK from pre-leukemic iNUP98-KMT2A mice and WT littermate controls. **P<0.01, unpaired t-test. (D) Competitive repopulation assay: lethally-irradiated CD45.1 WT recipients were transplanted with a 1:1 mixture of total BM cells from CD45.2 iNUP98-KMT2A and CD45.1 WT mice in the presence of doxycycline (DOX). The percentage of CD45.2 cells present in the peripheral blood was measured by flow cytometry over a period of 25 weeks. *P<0.05, unpaired t-test. (E) At week 25 of the competitive repopulation assay, the CD45.2+ cells were analyzed for percentages of Gr-1, CD3, and B220 markers. (F) Cellular BM chimerism of WT CD45.1+ mice 25 weeks after competitive transplantation with CD45.2+ iNUP98-KMT2A BM. All mice were exposed to DOX throughout the experiment.
leukemic phenotype characterized by the presence of leukemic blasts on peripheral blood smears, and exten- sive leukemic infiltration in the BM, spleen, liver and lungs (Figure 3A, Online Supplementary Figure S2A). This was accompanied by significantly increased white blood cell counts (P=0.0018, unpaired t-test, n=5) and abnormal leukocytes (“LUC”) (P=0.0468, unpaired t-test, n=5) counts in the periphery and splenomegaly (Figure 3B, Online Supplementary Table S4). Immunophenotypic analysis of highly-infiltrated BM revealed intermediate to high expression levels of myeloid markers Mac-1, Gr-1, and FcgRII/III in five out of six mice, with baseline expres- sion of B220 and CD3 lymphoid markers characterizing the disease as AML (Figure 3C, Online Supplementary Figure S2B-D).
Transplantation of total BM from diseased mice into sublethally-irradiated WT mice induced disease in 100% of recipients. Whereas disease development was fully doxycycline-dependent upon transplantation of AML
cells from one donor (“M1”) (median latency 28 weeks, P=0.025, log-rank test, n=4), leukemic cells from another donor (“M2”) resulted in disease in all recipients regard- less of doxycycline administration, with a similar latency (26-37 weeks) to that of recipients of “M1” cells on doxy- cycline (Figure 3D). Flow cytometric analysis of BM sam- ples from transplanted leukemic mice revealed hetero- geneity in M1 (“M1a” & “M1b”) BM recipients, including a marked doxycycline-dependent increase in CD3+ cells, but a consistent myeloid phenotype in recipients of M2 (Figure 3E).
Given the long latency to develop primary disease, we explored whether additional genotoxic insults might accelerate disease induction. Indeed, sublethal g-irradia- tion (1x 600 cGy) of asymptomatic 3- to 4-week old iNUP98-KMT2A mice on doxycycline resulted in earlier onset of disease symptoms than that observed in sub- lethally-irradiated WT mice (median latency 26 weeks vs. undefined; P=0.032, log-rank test, n=5) (Figure 3F, Online
haematologica | 2020; 105(7)
1861