Page 98 - Haematologica - Vol. 105 n. 6 - June 2020
P. 98
F. Stölzel et al.
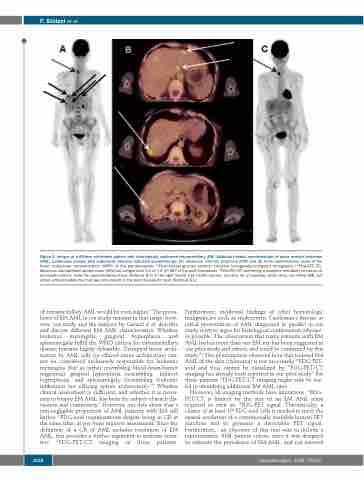
Figure 2. Images of a 69-year old female patient with histologically confirmed extramedullary (EM), bilobular hepatic manifestations of acute myeloid leukemia (AML) (continuous arrows) who underwent intensive induction chemotherapy. (A) Maximum intensity projection (MIP) and (B) three representative slices of the fused multiplanar reconstructions (MPR) of the pre-therapeutic 18Fluorodesoxy-glucose positron emission tomography/computed tomography (18FDG-PET/CT). Maximum standardized uptake value (SUVmax) ranged from 5.2 to 7.4. (C) MIP of the post-therapeutic 18FDG-PET/CT confirming a complete metabolic remission of all hepatic lesions. Note the hypermetabolic focus (SUVmax 8.9) in the right thyroid lobe (dotted arrows, see also (A) at baseline) which does not reflect AML but rather a thyroid adenoma that was still present in the post-therapeutic scan (SUVmax 8.1).
of extramedullary AML would be even higher.5 The preva- lence of EM AML in our study remains in that range; how- ever, our study and the analysis by Ganzel et al. describe and discuss different EM AML characteristics. Whether leukemic meningitis, gingival hyperplasia, and splenomegaly fulfill the WHO criteria for extramedullary disease remains highly debatable. Disrupted tissue archi- tecture by AML cells (or effaced tissue architecture) can- not be considered exclusively responsible for leukemic meningitis (but as rather resembling blood-brain-barrier migration), gingival hyperplasia (resembling indirect hyperplasia), and splenomegaly (resembling leukemic infiltration not effacing spleen architecture).1,20 Whether clinical assessment is sufficient, and whether it is neces- sary to biopsy EM AML, has been the subject of much dis- cussion and controversy.5 However, our data show that a non-negligible proportion of AML patients with EM still harbor 18FDG-avid manifestations despite being in CR at the same time, as per bone marrow assessment. Since the definition of a CR of AML includes resolution of EM AML, this provides a further argument to perform sensi- tive 18FDG-PET/CT imaging in these patients.
Furthermore, incidental findings of other hematologic malignancies such as multicentric Castleman’s disease at initial presentation of AML diagnosed in parallel in our study seem to argue for histological confirmation whenev- er possible. The observation that many patients with EM AML harbor more than one EM site has been suggested in our pilot study and others, and could be confirmed by this study.5,8 The phenomenon observed here that isolated EM AML of the skin (chloroma) is not necessarily 18FDG-PET- avid and thus cannot be visualized by 18FDG-PET/CT imaging has already been reported in our pilot study.8 For these patients 18FDG-PET/CT imaging might only be use- ful in identifying additional EM AML sites.
However, all imaging methods have limitations. 18FDG- PET/CT is limited by the size of an EM AML mass required to emit an 18FDG-PET signal. Theoretically, a cluster of at least 106 FDG avid cells is needed to meet the spatial resolution of a commercially available human PET machine and to generate a detectable PET signal. Furthermore, an objective of this trial was to include a representative AML patient cohort, since it was designed to estimate the prevalence of EM AML, and not survival
1556
haematologica | 2020; 105(6)