Page 42 - Haematologica - Vol. 105 n. 6 - June 2020
P. 42
G. Pavlasova and M. Mraz
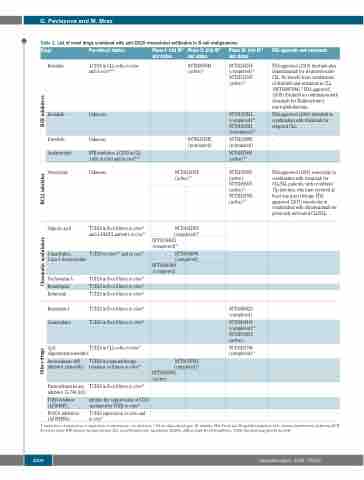
Table 2. List of novel drugs combined with anti-CD20 monoclonal antibodies in B-cell malignancies.
Drugs
Ibrutinib
Idelalisib
Duvelisib
Acalabrutinib
Venetoclax
Valproic acid
5-Azacitidine, 5-aza-2-deoxycytidine
Trichostatin A Romidepsin Entinostat
Pre-clinicalstudies
↓CD20 in CLL cells in vitro and in vivo44,100
Unknown
Unknown
BTK inhibition ↓CD20 in CLL cells in vitro and in vivo44,100
Unknown
↑CD20 in B-cell lines in vitro65 and in DLBCL patients in vivo78
↑CD20 in vitro75,76 and in vivo77
↑CD20 in B-cell lines in vitro74 ↑CD20 in B-cell lines in vitro65 ↑CD20 in B-cell lines in vitro81
PhaseI:trialID* and status
PhaseII:trialID* and status
NCT02007044 (active)12
NCT02391545 (terminated)
PhaseIII:trialID* and status
NCT02264574 (completed)49 NCT02165397 (active)118
NCT01539512 (completed)110 NCT01659021 (terminated)111
NCT02204982 (terminated)
FDAapprovalsandcomments
FDA approved (2019) ibrutinib plus obinutuzumab for treatment-naïve CLL. No benefit from combination of ibrutinib and rituximab in CLL (NCT02007044).12 FDA approved (2018) ibrutinib in combination with rituximab for Waldenström’s macroglobulinemia.
FDA approved (2014) idelalisib in combination with rituximab for relapsed CLL.
-
-
FDA approved (2018) venetoclax in combination with rituximab for CLL/SLL patients, with or without 17p deletion, who have received at least one prior therapy. FDA approved (2019) venetoclax in combination with obinutuzumab for previously untreated CLL/SLL.
Bryostatin-1
Gemcitabine
CpG oligodeoxynucleotides
Aurora kinase A/B inhibitor (alisertib)
Farnesyltransferase inhibitor (L-744, 832)
TGFβ inhibitor (LY364947)
↑CD20 in B-cell lines in vitro64 ↑CD20 in B-cell lines in vitro66
↑CD20 in CLL cells in vitro121 ↑CD20 in immunotherapy-
resistant cell lines in vitro123 ↑CD20 in B-cell lines in vitro62
inhibits the suppression of CD20
mediated by TGFβ in vitro99
FOXO1 inhibitors ↑CD20 expression in vitro and (AS1842856) in vivo70
NCT02296918 (active)119
NCT01622439 (completed)79
NCT02144623 (completed)80
NCT01004991
(completed) NCT00901069
(completed)
NCT02475681
(active)109
NCT02950051 (active) NCT02005471 (active)10 NCT02242942 (active)116
NCT01397825 (completed)72
NCT01695941
(active)
NCT00087425 (completed)
NCT00169195 (completed)120 NCT02750670 (active)
NCT00251394 (completed)122
↑: induction of expression; ↓: repression of expression; -: no decision; *: ID on clinicaltrials.gov.; ID: identity; FDA: Food and Drug Administration; CLL: chronic lymphocytic leukemia; BCR: B-cell receptor; BTK: Bruton tyrosine kinase; SLL: small lymphocytic lymphoma; DLBCL: diffuse large B-cell lymphoma; TGFβ: transforming growth factor-β.
1500
haematologica | 2020; 105(6)
Other drugs Chromatin modulators BCL2 inhibitor BCR inhibitors