Page 251 - Haematologica - Vol. 105 n. 6 - June 2020
P. 251
Hypercoagulation and relapse in breast cancer
In particular, we chose to study the role of fibrinogen, FVIIa-AT, F1+2, and D-dimer. Fibrinogen, a glycoprotein synthesized by the liver, is enzymatically converted to fib- rin by thrombin during the coagulation process. In normal conditions, it circulates in plasma at high concentrations, and its levels increase in inflammatory states as part of the acute-phase response. Circulating levels of FVIIa-AT com- plexes reflect the degree of exposure of cellular tissue fac- tor to blood; increased FVIIa-AT levels have been reported in a number of prothrombotic conditions, including solid and hematologic malignancies.23 F1+2 is a peptide released during the proteolytic activation of prothrombin into active thrombin, and represents a parameter of in vivo thrombin formation, while D-dimer is the primary degra- dation product of cross-linked fibrin, representing an index of both coagulation and fibrinolysis activation. It has been suggested that fibrinogen is involved in several stages of cancer progression.24 In vivo, in breast cancer patients, high fibrinogen levels have been associated with poorer overall survival25,26 and poor response to therapy,27 while increase in D-dimer levels has been related to growth rate, tumor burden, progression rate, and sur- vival.12,28
In the present prospective cohort of patients with resected tumors and candidates to adjuvant therapy, we found the occurrence of a moderate, but significant, hypercoagulable state, as indicated by the increased plas- ma levels of fibrinogen, F1+2, and D-dimer. The lack of correlation between thrombotic biomarker levels and the time from surgery suggests involvement of a tumor-relat- ed mechanism in the hypercoagulability observed in these patients. Indeed, levels of thrombotic biomarkers, and par- ticularly D-dimer and fibrinogen, positively and signifi- cantly correlated with tumor size and lymph node metas- tasis. The association with tumor characteristics might suggest an imprinting of the primary tumor on the coagu- lation system after resection, and might also represent the activity of residual occult circulating tumor cells on blood coagulation. In fact, it is well known that breast cancer cells can activate blood coagulation through several mech- anisms,4,29,30 and in vivo studies in breast cancer patients show significant correlations between increased D-dimer levels with circulating tumor cells and number of metasta- sis,31 as well as with lymphovascular invasion, clinical stage, and lymph node involvement.32
To understand the relevance of our observation in rela-
Table 3. Plasma levels of coagulation biomarkers according to disease recurrence.
Table 2. Hematologic parameters in the study subjects.
Patients
Whitebloodcells(x109/L) 6.8(2.0)
Redbloodcells(x1012/L) 4.47(0.51)
Reference range
(4.2-9.4)
(4.7-5.82)
(14-17) (150-400)
Out of range
4%(<4.2) 9% (>9.4)
76%(<4.7)
0.5% (>5.82)
75% (<14)
5% (>400)
D-dimer (ng/mL)
FVIIa-AT (pM)
F 1+2 (pmol/L)
Fibrinogen (mg/dL)
No-DR
196 (49-647)
120 (70-269)
197 (117-385)
298 (211-489)
DR
224 (30-653)
110 (62-335)
219 (114-628)
321 (209-455)
P
0.270
0.382
0.024
0.909
Hemoglobin (g/dL) Platelets (x109/L)
13.1 (1.3) 267 (80)
Data are shown as median and range (5th-95th percentiles). P-value calculated by Mann-Whitney test. AT: antithrombin; F 1+2: prothrombin fragment 1+2; DR: disease recurrence.
Data of patients are shown as mean (standard deviation). Reference ranges are inter- nally defined.
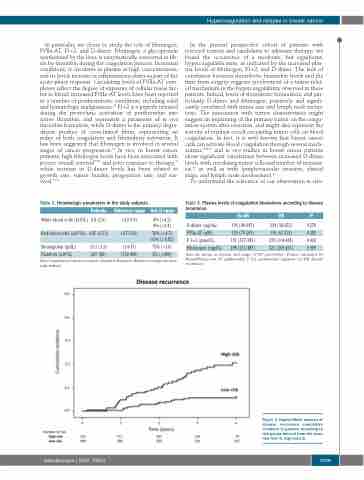
Figure 3. Kaplan-Meier analysis of disease recurrence cumulative incidence in patients according to risk-groups derived from the score (low-risk<3, high-risk≥3).
haematologica | 2020; 105(6)
1709