Page 23 - Haematologica - Vol. 105 n. 6 - June 2020
P. 23
Immunoregulatory functions of CD71+ erythroid cells
How erythroid precursors modulate immune responses in cancer
Anemia has been described as a primary consequence of tumor development in some oncological patients and ani- mal models of cancer.31 The pathogenesis of cancer-related anemia is complicated and can be multifactorial. There are several reports on EE development in malignant solid tumors such as breast and lung cancers.32,33 Although the principal explanation for the formation of EE in solid tumors is unknown, it appears that erythropoiesis-stimu- lating agents may play a pivotal role in the emergence of EE niches in cancer patients.34 A recent study highlighted the expansion of CEC (named Ter-cells in that study) in the spleen of an animal model of hepatocellular carcino- ma.35 Han et al. reported that tumor-derived transforming growth factor (TGF)-β activates the Smad3 downstream signaling pathway, which induces CEC from erythroid progenitor cells in the spleen. These CEC, by releasing artemin, a member of the glial cell line-derived neu- rotrophic factor family, directly promote the development and metastasis of hepatocellular carcinoma via an interac- tion of artemin with its receptor GFRα3 on tumor cells.35 Although Han et al. claimed that these erythroid precur- sors lacked immunosuppressive properties, a more recent study demonstrated immunosuppressive effects of CEC in advanced cancer.36 The latter study reported an association
between the expansion of immunosuppressive CEC and impaired Epstein-Barr virus-specific CD8+ T-cell prolifera- tion in patients with advanced cancer who were anemic.36 This research group also described strongly impaired CD8+ and CD4+ T-cell proliferation from melanoma-bear- ing C57BL/6 mice by splenic CEC when co-cultured in vitro,36 and that CD45+-expressing CEC showed more robust production of reactive oxygen species (ROS) com- pared to CD45- CEC.36 Accordingly, CD45+ CEC exhibited more potent suppression of virus-specific CD8+ T-cell pro- liferation compared to their CD45- counterparts in a mouse model of chronic lymphocytic choriomeningitis virus infection.36
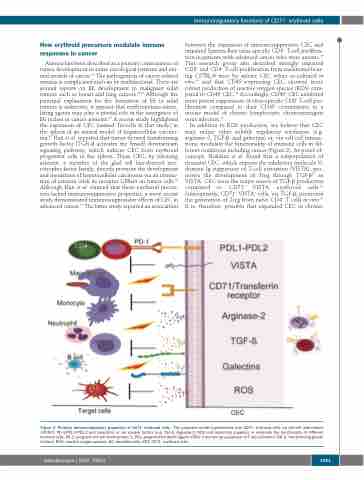
In addition to ROS production, we believe that CEC may utilize other soluble regulatory mediators (e.g. arginase-2, TGF-β, and galectins) or, via cell-cell interac- tions, modulate the functionality of immune cells in dif- ferent conditions including cancer (Figure 2). As proof-of- concept, Shahbaz et al. found that a subpopulation of neonatal CEC, which express the inhibitory molecule V- domain Ig suppressor of T-cell activation (VISTA), pro- motes the development of Treg through TGF-β37 as VISTA+ CEC were the major source of TGF-β production compared to CD71+ VISTA- erythroid cells.37 Subsequently, CD71+ VISTA+ cells, via TGF-β, promoted the generation of Treg from naïve CD4+ T cells in vitro.37 It is, therefore, possible that expanded CEC in chronic
Figure 2. Putative immunoregulatory properties of CD71+ erythroid cells. The proposed model hypothesizes that CD71+ erythroid cells via cell-cell interactions (VISTA:?, PD-1:PDL1/PDL-2 and Galectins) or via soluble factors (e.g. TGF-β, Arginase-2, ROS and Galectins) suppress or modulate the functionality of different immune cells. PD-1: programmed cell death protein 1; PDL: programmed death ligand; VISTA: V-domain Ig suppressor of T-cell activation; TGF-β: transforming growth factor-β. ROS: reactive oxygen species; DC: dendritic cells; CEC: CD71+ erythroid cells.
haematologica | 2020; 105(6)
1481