Page 22 - Haematologica - Vol. 105 n. 6 - June 2020
P. 22
E. Elahi and S. Mashhouri
sues of healthy mothers exhibited immunosuppressive properties. However, the frequency of CEC was lower in mothers with inflammatory bowel disease (IBD) and the cells were functionally impaired when examined in vitro.13 IBD is associated with intestinal dysbiosis and dysfunc- tional interaction between the microbiota and the gut mucosal immune system, which results in a dysregulated immune response against commensal microbial anti- gens.23 The reduced frequency and/or impaired function- ality of CEC during pregnancy may, therefore, predispose patients with IBD to a more pro-inflammatory milieu in their gastrointestinal tract, characterized by lower num- bers of regulatory T cells (Treg), higher concentrations of IL-6 and TNF-α, and dysbiosis.13 In line with this, in the absence of CEC, upregulation of IL-6 and TNF-α produc- tion by residential antigen-presenting cells in the gut was observed in a mouse model of pregnancy.13,22 Immune activation following upregulation of TNF-α production may result in excessive tissue damage and disruption of tight junctions in IBD mothers.24 In agreement with this, an increased permeability of the intestinal epithelial bar- rier was noted in pregnant mice when CEC were deplet- ed.13 Compromised intestinal barrier integrity may result in translocation of bacteria and their products, triggering a vicious cycle of inflammatory response.
An inflammatory milieu may influence the diversity and frequency of microbial communities in the gut. As such,
A
B
the ablation of CEC was associated with dysbiosis during pregnancy, suggesting a crucial role for these cells in main- taining homeostasis and symbiosis.13 The initial establish- ment of the neonatal microbiome is mainly determined by maternal-newborn exchanges of the microbiota. During normal vaginal delivery, the newborn is exposed to an army of new allies, which colonize the urogenital tract of the mother.25 Delivery via Cesarean section deprives the newborn of these microbial communities.26 Thus, vaginal delivery and subsequent exposure to maternal microbiota via nursing are evolutionarily important to the develop- ment of the newborn’s immune system. Interestingly, not only was the frequency of CEC observed in the cord blood and placenta of a twin delivered by Cesarean section lower than that of the vaginally delivered twin, but the CEC of the two twins also had a different gene profile.27 It can, therefore, be speculated that lower levels of CEC in IBD patients may result in intestinal dysbiosis and poor preg- nancy outcomes.28,29 In support of this hypothesis, lower CEC frequency was associated with preterm deliveries and emergency Cesarean sections.30 Therefore, during gesta- tion EE is not only required as a physiological response to the demand for a greater blood supply but it also plays a crucial role in feto-maternal tolerance and symbiosis. However, whether the source of abundant CEC in preg- nant women, similar to mice, is splenic EE or due to pas- sive incontinence from the bone marrow is unknown.
C
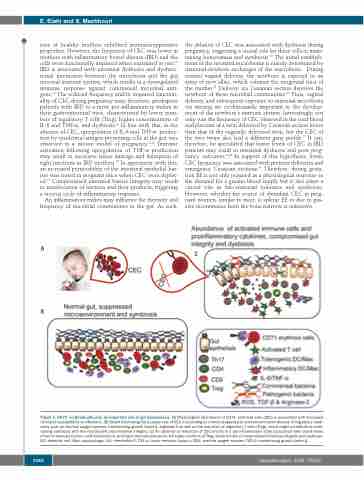
Figure 1. CD71+ erythroid cells play an important role in gut homeostasis. (A) Physiological abundance of CD71+ erythroid cells (CEC) is associated with increased neonatal susceptibility to infections. (B) Model illustrating the putative role of CEC in providing an immunosuppressive environment upon release of regulatory medi- ators such as reactive oxygen species, transforming growth factor-β, arginase-2 as well as the induction of regulatory T cells (Treg), which might contribute to main- taining symbiosis with the microbiome and intestinal integrity. (C) An absence or reduction of CEC results in a pro-inflammatory state associated with raised levels of tumor necrosis factor-α and interleukin-6, and hyper-immune activation, but lower numbers of Treg, which results in compromised intestinal integrity and dysbiosis. DC: dendritic cell; Mac; macrophage; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; ROS: reactive oxygen species; TGF-β: transforming growth factor-β.
1480
haematologica | 2020; 105(6)