Page 211 - Haematologica - Vol. 105 n. 6 - June 2020
P. 211
Cotl1 regulates shear-dependent thrombus formation
ABC
DEF
G
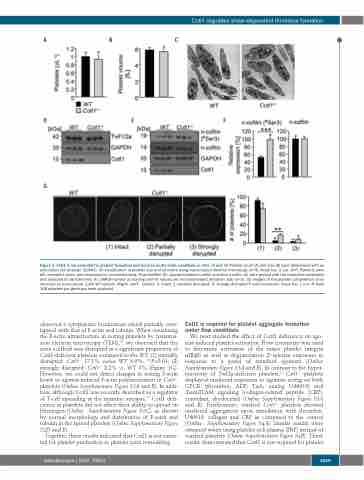
Figure 1. Cotl1 is not essential for platelet formation and function under static conditions in vitro. (A and B) Platelet count (A) and size (B) were determined with an automated cell analyzer (ScilVet). (C) Visualization of platelet size and structure using transmission electron microscopy (n=4). Scale bar, 2 μm. (D-F) Platelets were left untreated, lysed, and processed for immunoblotting. Total twinfilin (D), phosphorylated n-cofilin and total n-cofilin (E) were probed with the respective antibodies and analyzed by densitometry (F). GAPDH served as loading control. Values are mean±standard deviation (SD) (n=3). (G) Images of the platelet cytoskeleton ultra- structure on poly-L-lysine. (Left) WT sample. (Right) Cotl1-/- sample. 0: intact, 1: partially disrupted, 2: strongly disrupted F-actin structures. Scale bar, 1 μm. At least 158 platelets per genotype were analyzed.
We next studied the effect of Cotl1 deficiency on ago- nist-induced platelet activation. Flow cytometry was used to determine activation of the major platelet integrin αIIbβ3 as well as degranulation (P-selectin exposure) in
observed a cytoplasmic localization which partially over-
lapped with that of F-actin and tubulin. When visualizing
the F-actin ultrastructure in resting platelets by transmis-
sion electron microscopy (TEM),23 we observed that the
actin scaffold was disrupted in a significant proportion of
Cotl1-deficient platelets compared to the WT: (1) partially
disrupted: Cotl1-/- 17.1% versus WT 4.9%, **P>0.01; (2) response to a panel of standard agonists (Online
strongly disrupted: Cotl1-/- 8.2% vs. WT 3% (Figure 1G). However, we could not detect changes in resting F-actin levels or agonist-induced F-actin polymerization in Cotl1-/- platelets (Online Supplementary Figure S2A and B). In addi- tion, although Cotl1 was recently described as a regulator of T-cell spreading at the immune synapse,14 Cotl1 defi- ciency in platelets did not affect their ability to spread on fibrinogen (Online Supplementary Figure S2C), as shown by normal morphology and distribution of F-actin and tubulin in the spread platelets (Online Supplementary Figure S2D and E).
Together, these results indicated that Cotl1 is not essen- tial for platelet production or platelet actin remodeling.
Supplementary Figure S3A and B). In contrast to the hyper- reactivity of Twf2a-deficient platelets,11 Cotl1-/- platelets displayed unaltered responses to agonists acting on both GPCR (thrombin, ADP, TxA2 analog U46619) and (hem)ITAM signaling (collagen-related peptide (CRP), convulxin, rhodocytin) (Online Supplementary Figure S3A and B). Furthermore, washed Cotl1-/- platelets showed unaltered aggregation upon stimulation with thrombin, U46619, collagen and CRP as compared to the control (Online Supplementary Figure S4A). Similar results were obtained when using platelet-rich plasma (PRP) instead of washed platelets (Online Supplementary Figure S4B). These results demonstrated that Cotl1 is not required for platelet
Cotl1 is required for platelet aggregate formation under flow conditions
haematologica | 2020; 105(6)
1669