Page 132 - Haematologica - Vol. 105 n. 6 - June 2020
P. 132
F. Drieux et al.
quickly (48 hours), only requires equipment already in reg- ular use, including a thermocycler and genetic fragment size analyzer. The profiles are publicly accessible and easy to interpret through a dedicated website. Finally, the assay is cost-effective (approximately $5 per sample).23 RT- MLPA is a useful tool in combination with pathological evaluation to characterize PTCL, especially when immunohistochemistry is flawed or incomplete. In addi- tion to evaluating the expression of Th-differentiation anti- gens and markers suitable for immunohistochemistry, the current RT-MLPA assay also provides genetic information, such as RHOA and IDH2 mutations, which are highly rel- evant for the diagnosis of PTCL of TFH origin,19 even though the RHOA G17V mutation has also been reported in a small minority of ATLL,25 as observed in one case of our series. The accurate classification of specified PTCL other than NOS entities in most cases corroborates the rel- evance of the designed gene panel. Altogether, although some markers in our RT-MLPA assay might not be useful in every PTCL case, this “one fits all” assay evaluates diag-
nostic markers covering the different PTCL entities in a systematic and cost-effective way.
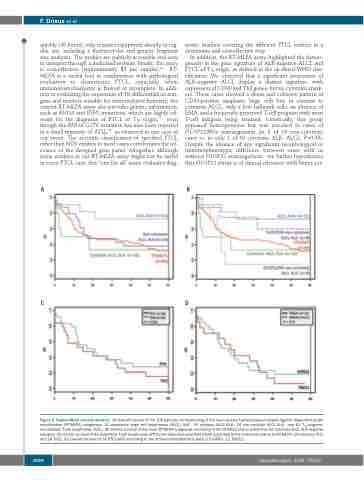
In addition, the RT-MLPA assay highlighted the hetero- geneity in the gene signature of ALK-negative ALCL and PTCL of TFH origin, as defined in the up-dated WHO clas- sification. We observed that a significant proportion of ALK-negative ALCL display a distinct signature, with expression of CD30 and Th2 genes, but no cytotoxic mark- ers. These cases showed a dense and cohesive pattern of CD30-positive anaplastic large cells but, in contrast to common ALCL, only a few hallmark cells, an absence of EMA, and a frequently preserved T-cell program with most T-cell antigens being retained. Genetically, this group appeared heterogeneous but was enriched in cases of DUSP22/IRF4 rearrangement (in 8 of 16 non-cytotoxic cases vs. in only 1 of 10 cytotoxic ALK- ALCL; P=0.09). Despite the absence of any significant morphological or immunophenotypic difference between cases with or without DUSP22 rearrangement, we further hypothesize that DUSP22 status is of clinical relevance with better sur-
AB
CD
Figure 5. Kaplan-Meier survival analysis. (A) Overall survival of the 108 patients corresponding of the main reverse transcriptase-multiplex ligation-dependent probe amplification (RT-MLPA) subgroups: 11 anaplastic large cell lymphomas (ALCL) ALK+, 10 cytotoxic ALCL ALK-, 24 non-cytotoxic ALCL ALK-, and 63 TFH/angioim- munoblastic T-cell lymphomas (AITL). (B) Overall survival of the main RT-MLPA subgroups according to the DUSP22 status within the non-cytotoxic ALCL ALK-negative category. (C) Overall survival of 43 peripheral T-cell lymphomas (PTCL)-not otherwise specified (NOS) according to the molecular status by RT-MLPA (19 cytotoxic/TH1 and 24 TH2). (D) Overall survival of 30 PTCL-NOS according to the immunohistochemistry data (19 GATA3, 11 TBX21).
1590
haematologica | 2020; 105(6)