Page 114 - Haematologica - Vol. 105 n. 6 - June 2020
P. 114
F. Ravandi et al.
Table 4. Pharmokinetic parameters for total arsenic.
ORH-2014 ORH-2014 ORH-2014 IV ATO
5 mg** 10 mg 15 mg 0.15 mg/kg
Day1 Day15 Day1 Day15 Day22 Day1 Day15 Day22 Day8
Cmax, ng/mL
AUC0-24, ng•h/mL
Tmax, h†
RA
(n=3)
7.22 (9.5) 125 (1.3)‡ 12.00 [12.0, 24.0] NA
(n=3)
34.0 (16.9) 729 (21.9) 8.10 [4.0, 8.18] 5.14
(1.4)‡
(n=6)
19.9 (36.8) 329 (32.7) 2.13 [2.00, 24.3] NA
(n=5)
65.6
(37.2)
1340
(37.6)
2.02
[1.00, 8.08]
4.19
(20.7)
(n=2)
109
(37.0)
2210
(41.2)
1.02
[0.97, 1.07]
5.27
(40.3)
(n=3)
24.5 (39.6) 454 (36.1) 3.85 [0.98, 24.0] NA
(n=3)
114
(21.1)
2140
(35.8)
1.00
[0.90, 1.13]
4.72
(5.9)
(n=3)
111 124 (29.5) (60) 2240 1302 (40.4) (30)
1.00 − [0.00, 1.95]
4.92 (5.5) −
See the Online Supplementary Materials and Methods for PK sampling and data analyses. Geometric mean (%CV) data are presented, unless otherwise noted. *Bolded cells are historical values for IV ATO (Trisenox®) calculated from data in NDA #21-248. ** protocol was amended to add a day 22 after the second dose cohort. †Median [min, max]. ‡N = 2. Cmax: maximum observed concentration; AUC0-24: area under the plasma drug concentration-time curve from 0 to 24 hours; Tmax: amount of time that the drug is present at the maximum concentration in serum, RA: ratio of accumulation; NA: not analyzed.
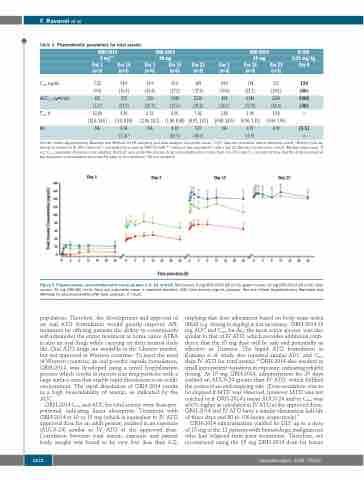
Figure 3. Plasma arsenic concentration-time curves at days 1, 5, 15, and 22. Red curves: 5 mg ORH-2014 QD (n=3); green curves: 10 mg ORH-2014 QD (n=6); blue curves: 15 mg ORH-QD (n=3). Data are arithmetic mean ± standard deviation (SD) total arsenic (ng/mL plasma). See the Online Supplementary Materials and Methods for pharmacokinetic (PK) data analyses. h: hours.
population. Therefore, the development and approval of an oral ATO formulation would greatly improve APL treatment by offering patients the ability to conveniently self-administer the entire treatment at home (since ATRA is also an oral drug) while carrying on their normal daily life. Oral ATO drugs are available in the Chinese market, but not approved in Western countries. To meet the need of Western countries, an oral powder capsule formulation, ORH-2014, was developed using a novel lyophilization process which results in micron-size drug particles with a large surface area that enable rapid dissolution in an acidic environment. The rapid dissolution of ORH-2014 results in a high bioavailability of arsenic, as indicated by the AUC.
ORH-2014 Cmax and AUC for total arsenic were dose-pro- portional, indicating linear absorption. Treatment with ORH-2014 at 10 or 15 mg (which is equivalent to IV ATO approved dose for an adult person) resulted in an exposure (AUC0-24) similar to IV ATO at the approved dose. Correlation between total arsenic exposure and patient body weight was found to be very low (less than 0.2),
implying that dose adjustment based on body mass index (BMI) (e.g. dosing in mg/kg) is not necessary. ORH-2014 15 mg AUC and Cmax for As3, the most active species, was also similar to that of IV ATO, which provides additional confi- dence that the 15 mg dose will be safe and potentially as effective as Trisenox. The liquid ATO formulation in Kumana et al. study also reported similar AUC and Cmax than IV ATO for total arsenic.19 ORH-2014 also resulted in small inter-patient variations in exposure, indicating reliable dosing. At 15 mg ORH-2014, administration for 25 days yielded an AUC0-24 greater than IV ATO, which fulfilled the protocol second stopping rule. (Dose-escalation was to be stopped if MTD was observed, however MTD was not reached or if ORH-2014’s mean AUC0-24 and/or Cmax was ≥30% higher as calculated in IV ATO at the approved dose). ORH-2014 and IV ATO have a similar elimination half-life of three days and 80 to 100 hours, respectively).9
ORH-2014 administration yielded no DLT up to a dose of 15 mg in the 12 patients with hematologic malignancies who had relapsed from prior treatments. Therefore, we recommend using the 15 mg ORH-2014 dose for future
1572
haematologica | 2020; 105(6)