Page 113 - Haematologica - Vol. 105 n. 6 - June 2020
P. 113
Oral arsenic trioxide pharmacokinetics and safety
Methods). Peak plasma concentrations of AsIII, the primary active species, were reached at approximately two hours across all doses (range: 1-4 hours). Plasma concentration of AsIII declined in a biphasic manner with a mean elimina- tion half-life of 7 to 16 hours and is characterized by an initial rapid distribution phase followed by a slower termi- nal elimination phase. Twenty-two days after daily administration of ORH-2014 at 15 mg, AsIII Cmax was 25 ng/mL (42% CV) and AUC was 354 ng*hr/mL (47% CV), which is comparable to the AUC of Trisenox (332 ng*hr/mL; n=6; on Day 25).9 After administration at 5, 10 or 15 mg on a daily regimen, accumulation of AsIII ranged from 1.05 to 1.44 compared to a single dose. The primary pentavalent metabolites, MMAV and DMAV, were slow to appear in plasma (approximately 4 to 24 hours after first administration of ORH-2014), but, due to their appar- ent longer half-life, accumulate more upon multiple dos- ing than does AsIII. The extent of accumulation of these metabolites is dependent on the dosing regimen. Accumulation ranged from 1.4- to 11-fold following mul- tiple dosing as compared to single dose administration. AsV is present in plasma only at relatively low levels. ORH-2014 AUC of As3 species, is comparable to the AsIII AUC of IV ATO.9
Efficacy
Although this phase 1 study was not intended to for- mally determine efficacy of ORH-2014, disease improve-
ments were observed in two patients with advanced MDS. One patient had complete marrow remission observed approximately 12 and 27 weeks after starting ORH-2014. The patient took 171 doses and was on the study for 187 days of ORH-2014 at 5 mg daily. Another patient had improvement in peripheral counts and became eligible for bone marrow transplant after 110 days of ORH-2014 dosing at 15 mg daily (this patient did not have any bone marrow procedures performed beyond the 30- day timepoint, to avoid an invasive procedure).
Discussion
In the past 5 years, the standard-of-care for non-high- risk APL patients has shifted from the combination of ATRA plus anthracycline chemotherapy to the combina- tion of IV ATO plus ATRA, triggering new treatment guidelines.2 However, IV ATO plus ATRA treatment regi- men consists of daily IV administration of ATO for over 100 days, which represents an important burden to patients and caregivers, and can be associated with low treatment compliance, low quality of life, and high costs. Poor compliance is an important issue with IV ATO treat- ment due to the burden of attending lengthy daily clinic visits for months, particularly for patients who live far from the treatment center, who work, and/or have family obligations, which is common in relatively younger AML
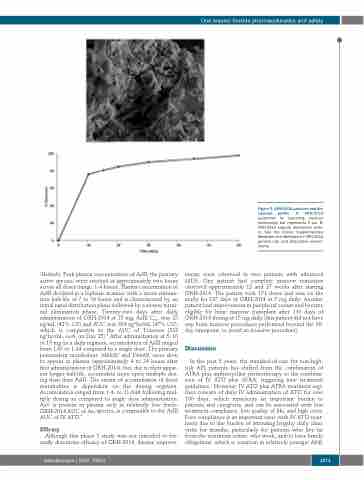
Figure 2. ORH-2014 particles and dis- solution profile. A: ORH-2014 Lyopremix by scanning electron microscopy; bar represents 5 μm. B: ORH-2014 capsule dissolution kinet- ic. See the Online Supplementary Materials and Methods for ORH-2014 particle size and dissolution assess- ments.
haematologica | 2020; 105(6)
1571