Page 76 - Haematologica May 2020
P. 76
N.U. Stoffel et al.
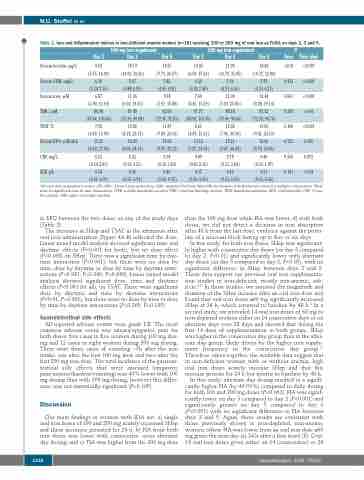
Table 2. Iron and inflammatory indices in iron-deficient anemic women (n=19) receiving 100 or 200 mg of oral iron as FeSO4 on days 2, 3 and 5.
Serum ferritin, mg/L1 Serum sTfR, mg/L2 Serum iron, mM TIBC, mM
TSAT, %
Serum EPO, mIU/mL
CRP, mg/L
AGP, g/L
9.62 (5.75, 16.09) 6.10 (5.24-7.16) 6.87 (3.90, 12.10) 86.68 (74.64, 100.66) 7.92 (4.49, 13.99) 15.35 (8.60, 27.38) 0.53 (0.24-2.83) 0.54
(0.32, 0.91)
18.19 (10.93, 30.26) 5.67 (4.88-6.29) 11.26 (6.56, 19.33) 81.49 (73.91, 89.84) 13.82 (8.18, 23.37) 15.09 (8.09, 28.13) 0.52 (0.21-3.21) 0.56
(0.35, 0.91)
Day5
14.35 (7.73, 26.67) 5.82 (4.69-6.91) 9.74 (5.97, 15.88) 82.00 (72.45, 92.81) 11.87 (7.00, 20.13) 17.02 (9.95, 29.12) 0.38 (0.25-1.20) 0.46
(0.29, 0.72)
Day2
10.02
(6.59, 15.26)
6.35
(4.92-7.49)
7.69
(4.45, 13.29)
91.71
(80.96, 103.90)
8.61
(4.87, 15.21)
15.12
(7.87, 29.04)
0.89
(0.43-2.12)
0.57
(0.36, 0.90)
Day5
18.46
(10.37, 32.84)
5.58
(4.51-6.25)
12.44
(8.08, 19.14)
83.12
(72.93, 94.74)
15.06
(9.41, 24.10)
16.43
(8.74, 30.86)
0.46
(0.23, 1.97)
0.31
(0.16, 0.62)
<0.01
0.413
0.061
0.268
0.106
0.727
0.556
0.101
<0.001
<0.001
<0.001
<0.01
<0.001
0.590
0.072
<0.01
Day2
100 mg iron supplement Day3
200 mg iron supplement Day3
21.99
(13.79, 35.05)
5.16
(4.71-6.66)
12.09
(5.65, 25.85)
84.38
(73.66, 96.66)
15.68
(7.96, 30.90)
18.21
(9.67, 34.29)
0.78
(0.31, 2.84)
0.46
(0.23, 0.93)
P
Dose Time(day)
1All such data as geometric means (-SD,+SD).2All such data as medians (IQR).Analyzed by Linear Mixed Model Analysis with Bonferroni corrected multiple comparisons. There were no significant dose by time interactions. sTFR: soluble transferrin receptor; TIBC: total iron binding capacity; TSAT: transferrin saturation; EPO: erythropoietin; CRP: C-reac- tive protein; AGP: alpha-1-acid glycoprotein.
in EPO between the two doses on any of the study days (Table 2).
The increases in SHep and TSAT in the afternoon after oral iron administration (Figure 4A-B) reflected the dose. Linear mixed model analysis showed significant time and daytime effects (P<0.001 for both), but no dose effect (P=0.168) on SHep. There was a significant time by day- time interaction (P<0.001), but there were no dose by time, dose by daytime or dose by time by daytime inter- actions (P=0.981, P=0.390, P=0.940). Linear mixed model analysis showed significant dose, time and daytime effects (P<0.001 for all), on TSAT. There were significant dose by daytime and time by daytime interactions (P<0.01, P<0.001), but there were no dose by time or dose by time by daytime interactions (P=0.265, P=0.185).
Gastrointestinal side effects
All reported adverse events were grade I-II. The most common adverse event was nausea/epigastric pain for both doses: five cases in five women during 100 mg dos- ing and 12 cases in eight women during 200 mg dosing. There were three cases of vomiting ~5h after the iron intake: one after the first 100 mg dose and two after the first 200 mg iron dose. The total incidence of the gastroin- testinal side effects that were assessed (epigastric pain/nausea/diarrhea/vomiting) was 40% lower with 100 mg dosing than with 200 mg dosing, however this differ- ence was not statistically significant (P=0.105).
Discussion
Our main findings in women with IDA are: a) single oral iron doses of 100 and 200 mg acutely increased SHep and these increases persisted for 24 h; b) FIA from both iron doses was lower with consecutive versus alternate day dosing; and c) TIA was higher from the 200 mg dose
than the 100 mg dose while FIA was lower; d) with both doses, we did not detect a decrease in iron absorption after 48 h from the last dose, evidence against the postu- late of a mucosal block lasting up to five or six days.
In this study, for both iron doses, SHep was significant- ly higher with consecutive day doses (on day 3 compared to day 2, P<0.01) and significantly lower with alternate day doses (on day 5 compared to day 3, P<0.05), with no significant difference in SHep between days 2 and 5. These data support our previous oral iron supplementa- tion studies in iron-deficient, mostly non-anemic, sub- jects.8,17 In those studies, we assessed the magnitude and duration of the SHep increase after an oral iron dose and found that oral iron doses ≥60 mg significantly increased SHep at 24 h, which returned to baseline by 48 h.8 In a second study, we provided 14 oral iron doses of 60 mg to iron-depleted women either on 14 consecutive days or on alternate days over 28 days and showed that during the first 14 days of supplementation in both groups, SHep was higher in the consecutive day group than in the alter- nate day group, likely driven by the higher iron supple- ment frequency in the consecutive day group.17 Therefore, taken together, the available data suggest that in iron-deficient women with or without anemia, high oral iron doses acutely increase SHep and that this increase persists for 24 h but returns to baseline by 48 h.
In this study, alternate day dosing resulted in a signifi- cantly higher FIA (by 40-50%) compared to daily dosing for both 100 and 200 mg doses (P<0.001). FIA was signif- icantly lower on day 3 compared to day 2 (P<0.001) and significantly greater on day 5 compared to day 3 (P<0.001) with no significant difference in FIA between days 2 and 5. Again, these results are consistent with those previously shown in iron-depleted, non-anemic women, where FIA was lower from an oral iron dose ≥60 mg given the next day (at 24 h after a first dose) (8). Over 14 oral iron doses given either on 14 (consecutive) or 28
1236
haematologica | 2020; 105(5)