Page 34 - Haematologica May 2020
P. 34
N. Curto-Garcia et al.
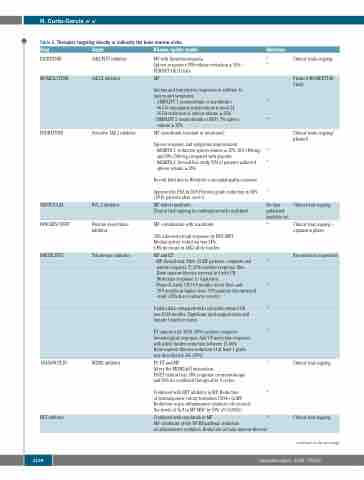
Table 2. Therapies targeting directly or indirectly the bone marrow niche.
Drug
PACRITINIB
MOMELOTINIB
FEDRATINIB
NAVITOCLAX
PANOBINOSTAT
IMETELSTAT
Target
JAK2/FLT3 inhibitor
JAK1/2 inhibitor
Selective JAK 2 inhibitor
BCL-2 inhibitor
Histone deacetylase inhibitor
Telomerase inhibitor
Disease/update results
MF with thrombocytopenia
Spleen responses 18% volume reduction ≥ 35% – PERSIST I & II trials
MF
Anemia and transfusion responses in addition to spleen and symptoms:
- SIMPLIFY 1 (momelotinib vs ruxolitinib):
Reference
82 83
84
85
86
87
99
No data published available yet 119
92
93
94
91
95
96
120
Clinical trials ongoing
Planned MOMENTUM Study
Clinical trials ongoing/ planned
Clinical trial ongoing
Clinical trial ongoing – expansion phase.
Recruitment suspended
66.5.% transfusion independent at week 24.
26.5% reduction of spleen volume ≥ 35%
- SIMPLIFY 2 (momelotinib vs BAT): 7% spleen
volume ≥ 35%
MF (ruxolitinib resistant or intolerant)
Spleen response and symptoms improvement.
- JAKARTA-1: reduction spleen volume ≥ 35%, 36% (400mg)
IDASANUTLIN
BET inhibitor
MDM2 inhibitor
Approved by FDA in 2019.Fibrosis grade-reduction in 44% (8/18) patients after cycle 6.
MF failed ruxolitinib.
Clinical trial ongoing in combination with ruxolitinib
MF -combination with ruxolitinib
36% achieved overall response by IWG-MRT Median spleen reduction was 34%
6.8% decrease in JAK2 allele burden
MF and ET.
- MF clinical trial: Pilot- 33 MF patients- complete and
partial response 7( 21%) median response 18m. Bone marrow fibrosis reversal in 4 with CR. Molecular response 3 / 4 patients.
- Phase-II study: OS 19.9 months in low dose and
29.9 months in higher dose. 93% patients discontinued study (25% due to adverse events).
Update data compared with real world showed OS was 30.69 months. Significant myelosuppression and hepatic toxicity in some.
ET clinical trial: 16/18 (89%) achieve complete hematological response. And 7/8 molecular response with allele burden reduction between 15-66%.
Bone marrow fibrosis reduction of at least 1 grade was described in 4/6 (67%)
PV, ET and MF
Alters the MDM2/p53 interaction.
PV/ET clinical trial: 58% response on monotherapy and 50% for combined therapy after 6 cycles.
Combined with BET inhibitor in MF: Reduction
of hematopoietic colony formation CD34+ in MF. Reduction in pro-inflammatory cytokines (decreased the levels of IL-8 in MF MNC by 50% (P=0.0003))
Clinical trial ongoing
Clinical trial ongoing
and 40% (500-mg compared with placebo.
- JAKARTA-2: Second line study 55% of patients achieved
spleen volume ≥ 35%.
Recent hold due to Wernicke’s encephalopathy removed.
Combined with ruxolitinib in MF
MF- inhibition of the NF-KB pathway, reduction
of inflammatory cytokines. Reduction of bone marrow fibrosis.
continued on the next page
1194
haematologica | 2020; 105(5)