Page 256 - Haematologica May 2020
P. 256
N. Eaton et al.
Results
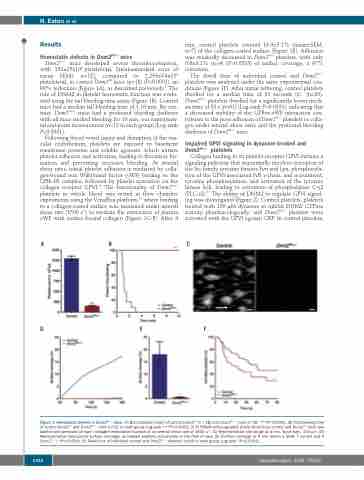
min, control platelets covered 18.0±5.1% (mean±SEM; n=7) of the collagen-coated surface (Figure 1E). Adhesion was markedly decreased in Dnm2Plt–/– platelets, with only 0.6±0.1% (n=4) (P=0.0333) of surface coverage, a 97% reduction.
The dwell time of individual control and Dnm2Plt–/– platelets was analyzed under the same experimental con- ditions (Figure 1F). After initial tethering, control platelets dwelled for a median time of 61 seconds (s) (n=60). Dnm2Plt–/– platelets dwelled for a significantly lower medi- an time of 33 s (n=61) (Log-rank P=0.0331), indicating that a decreased stability of the GPIbα-vWF interaction con- tributes to the poor adhesion of Dnm2Plt–/– platelets to colla- gen under arterial shear rates and the profound bleeding diathesis of Dnm2Plt–/– mice.
Impaired GPVI signaling in dynasore-treated and Dnm2Plt–/– platelets
Collagen binding to its platelet receptor GPVI initiates a signaling pathway that sequentially involves activation of the Src family tyrosine kinases Fyn and Lyn, phosphoryla- tion of the GPVI-associated FcR γ-chain, and recruitment, tyrosine phosphorylation, and activation of the tyrosine kinase Syk, leading to activation of phospholipase C-γ2 (PLC-γ2).27 The ability of DNM2 to regulate GPVI signal- ing was investigated (Figure 2). Control platelets, platelets treated with 100 mM dynasore to inhibit DNM2 GTPase activity pharmacologically, and Dnm2Plt–/– platelets were activated with the GPVI agonist CRP. In control platelets,
HemostaticdefectsinDnm2Plt–/– mice
Dnm2Plt–/– mice developed severe thrombocytopenia,
with 152±15x103 platelets/mL [mean±standard error of mean SEM); n=15], compared to 1,299±54x103 platelets/mL in control Dnm2fl/fl mice (n=18) (P<0.0001), an 88% reduction (Figure 1A), as described previously.9 The role of DNM2 in platelet hemostatic function was evalu- ated using the tail bleeding time assay (Figure 1B). Control mice had a median tail bleeding time of 1.16 min. By con- trast, Dnm2Plt–/– mice had a profound bleeding diathesis with all mice studied bleeding for 10 min, our experimen- tal end-point measurement (n=12 in each group) (Log-rank P<0.0001).
Following blood vessel injury and disruption of the vas- cular endothelium, platelets are exposed to basement membrane proteins and soluble agonists, which initiate platelet adhesion and activation, leading to thrombus for- mation and preventing excessive bleeding. At arterial shear rates, initial platelet adhesion is mediated by colla- gen-bound von Willebrand factor (vWF) binding to the GPIb-IX complex, followed by platelet activation via the collagen receptor GPVI.26 The functionality of Dnm2Plt–/– platelets in whole blood was tested in flow chamber experiments using the VenaFlux platform,20 where binding to a collagen-coated surface was measured under arterial shear rate (1500 s–1) to mediate the interaction of plasma vWF with surface-bound collagen (Figure 1C-F). After 4
ABC
DEF
Figure 1. Hemostatic defects in Dnm2Plt–/– mice. (A) Blood platelet count of control Dnm2fl/fl (n = 18) and Dnm2Plt–/– mice (n=15; ***P<0.0001). (B) Tail bleeding time of control Dnm2fl/fl and Dnm2Plt–/– mice (n=12 in each group; Log-rank ***P<0.0001). (C-F) PPACK-anticoagulated whole blood from control and Dnm2fl/fl mice was labeled and perfused on type I collagen-immobilized surface at an arterial shear rate of 1500 s–1. (C) Representative still image at 4 min. Scale bars, 100 μm. (D) Representative time-course surface coverage, as labeled platelets accumulate in the field of view. (E) Surface coverage at 4 min (mean ± SEM; 7 control and 4 Dnm2Plt–/–; *P=0.0333). (F) Dwell time of individual control and Dnm2Plt–/– platelets (n=60 in each group; Log-rank *P=0.0331).
1416
haematologica | 2020; 105(5)