Page 196 - Haematologica May 2020
P. 196
B. Mariotti et al.
A
B
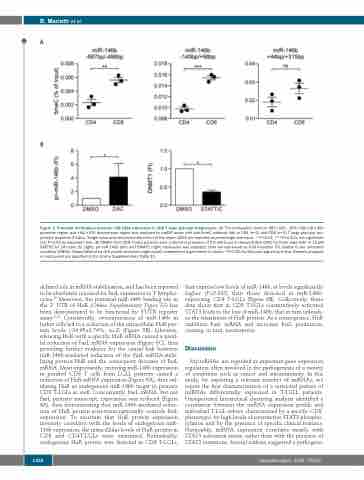
Figure 3. Promoter methylation prevents miR-146b expression in CD8 T large granular lymphocytes. (A) The methylation level of -687/-496, -149/+98 miR-146b promoter region and +44/+315 downstream region was analyzed by meDIP assay with anti-5meC antibody (Ab) in CD4 (n=3) and CD8 (n=3) T large granular lym- phocyte leukemia (T-LGLs). Single value and mean±standard error of the mean (SEM) are reported as percentage over input. **P<0.01, ***P<0.001, not significant (ns) P>0.05 by unpaired t-test. (B) PBMCs from CD8 T-LGLL patients were cultured in presence of 2.5 mM 5-aza-2’-deoxycytidine (DAC) for three days (left) or 15 μM STATTIC for 24 hours (h) (right). pri-miR-146b (left) and DNMT1 (right) expression was analyzed. Data are expressed as Fold Induction (FI) relative to the untreated condition (DMSO). Mean±SEM of six (left panel) and seven (right panel) independent experiments is shown. *P<0.05, by Wilcoxon signed-rank test. Patients analyzed in each panel are specified in the Online Supplementary Table S5.
defined role in mRNA stabilization, and has been reported to be absolutely required for FasL expression in T lympho- cytes.29 Moreover, the potential miR-146b binding site in the 3’ UTR of HuR (Online Supplementary Figure S3) has been demonstrated to be functional by 3’UTR reporter assay.30,31 Consistently, overexpression of miR-146b in Jurkat cells led to a reduction of the intracellular HuR pro- tein levels (-34.05±3.74%, n=2) (Figure 5B). Likewise, silencing HuR with a specific HuR siRNA caused a paral- lel reduction of FasL mRNA expression (Figure 5C), thus providing further evidence for the causal link between miR-146b-mediated reduction of the FasL mRNA-stabi- lizing protein HuR and the consequent decrease of FasL mRNA. Most importantly, restoring miR-146b expression in purified CD8 T cells from LGLL patients caused a reduction of HuR mRNA expression (Figure 6A), thus val- idating HuR as endogenous miR-146b target in primary CD8 T-LGLs as well. Concurrently, FasL mRNA, but not FasL primary transcript, expression was reduced (Figure 6A), thus demonstrating that miR-146b-mediated reduc- tion of HuR protein post-transcriptionally controls FasL expression. To ascertain that HuR protein expression inversely correlates with the levels of endogenous miR- 146b expression, the intracellular levels of HuR protein in CD8 and CD4T-LGLs were examined. Remarkably, endogenous HuR protein was detected in CD8 T-LGLs,
that express low levels of miR-146b, at levels significantly higher (P=0.003) than those detected in miR-146b- expressing CD4 T-LGLs (Figure 6B). Collectively, these data show that in CD8 T-LGLs constitutively activated STAT3 leads to the loss of miR-146b, that in turn unleash- es the translation of HuR protein. As a consequence, HuR stabilizes FasL mRNA and increases FasL production, causing, in turn, neutropenia.
Discussion
MicroRNAs are regarded as important gene expression regulators often involved in the pathogenesis of a variety of conditions such as cancer and autoimmunity. In this study, by exploring a relevant number of miRNAs, we report the first characterization of a restricted pattern of miRNAs differentially expressed in T-LGLL patients. Unsupervised hierarchical clustering analysis identified a correlation between the miRNA expression profile and individual T-LGL subset, characterized by a specific CD8+ phenotype, by high levels of constitutive STAT3 phospho- rylation and by the presence of specific clinical features. Noticeably, miRNA expression correlates mostly with STAT3 activation status, rather than with the presence of STAT3 mutations. Several authors suggested a pathogenic
1356
haematologica | 2020; 105(5)