Page 130 - Haematologica May 2020
P. 130
C. Yu et al.
pared to wt BECLIN-1, pointing to a BECLIN-1-specific autophagy regulation by BCR-ABL (Figure 4F-H).
In further BECLIN-1 complex analyses, we could demonstrate that the resistance of the phospho-mimic Beclin-1 mutant to nilotinib-induced autophagy is caused by an altered composition of the BECLIN-1 core complex with an impaired recruitment of the activation compo- nents ATG14, UVRAG, VPS15 and a gain of the negative regulator RUBICON to the BECLIN-1 core complex (Online Supplementary Figure S5A). BECLIN-1 phosphoryla- tion with subsequent resistance to TKI-induced autophagy may thereby provide a novel explanation of how leukemic cells can escape autophagy-induced cell death and develop TKI resistance.
Recently, it has been shown that BECLIN-1 S90 phos- phorylation is involved in starvation-mediated autophagy.39 To test whether BECLIN-1 phosphorylation at Y233/Y352 can influence starvation-mediated or rapamycin-mediated autophagy, we starved K562 cells or treated them with rapamycin and found that cellular autophagy is induced in Beclin-1 wt cells (Online Supplementary Figure S5B-D) and no differences could be demonstrated in K562 cells expressing either BECLIN-1 Y233E/Y352E or BECLIN-1 Y233F/Y352F (Online Supplementary Figure S5E-H). These results indicate that tyrosine phosphorylation of BECLIN-1 at Y233 and Y352 is not involved in starvation- or rapamycin-mediated autophagy but rather seems to be specific for tyrosine kinase-mediated autophagy processes.
Discussion
Recently, several studies have suggested that autophagy, a mechanism maintaining cellular homeostasis, plays an essential role in CML. However, the precise machinery of autophagy in CML development is not completely under- stood and crucial autophagocytotic mediators have not been investigated in vivo for their role in leukemogenesis in relevant CML mouse models.
Here, we define a molecular mechanism of autophagy suppression by BCR-ABL-specific BECLIN-1 phosphoryla- tion. Silencing of Beclin-1 by siRNA technology led to a significantly prolonged survival of BCR-ABL transplanted mice, whereas no profound differences could be found for Atg5 deletion. Binding of BECLIN-1 to BCR-ABL led to phosphorylation at tyrosine residues Y233 and Y352, alteration of the BECLIN-1 interactome, and suppression of autophagy function.
Several active oncogenic kinases were demonstrated to serve as negative regulators of autophagy processes, whereas inhibition of oncogenic tyrosine kinases can reverse this effect. Until now, some links of BCR-ABL to autophagy processes have been described: BCR-ABL acti- vates the PI3K/AKT signaling pathway, which is consid- ered as a pathway inhibiting autophagy. Furthermore, TKI treatment itself triggers autophagy in BCR-ABL+ cells and TKI-induced cell death can potentially be increased by tar- geting autophagy proteins in addition. Recently, it was demonstrated that Ponatinib-resistant CML cells can
AB
C
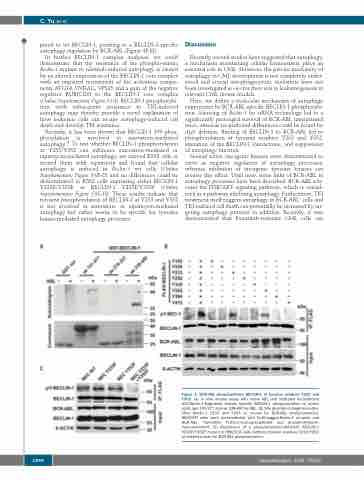
Figure 3. BCR-ABL phosphorylates BECLIN-1 at tyrosine residues Y233 and Y352. (A) In vitro kinase assay with active ABL and indicated recombinant GST-Beclin-1-fragments reveals specific BECLIN-1 phosphorylation at amino acids (aa) 141-277 and aa 338-450 by ABL. (B) Site directed mutagenesis iden- tifies Beclin-1 Y233 and Y253 as crucial for BCR-ABL phosphorylation. HEK293T cells were co-transfected with FLAG-tagged-Beclin-1 mutants and BCR-ABL, thereafter FLAG-immunoprecipitated and phosphotyrosine- immunoblotted. (C) Expression of a phosphorylation-deficient BECLIN-1 Y233F/Y352F mutant in HEK293T cells confirms tyrosine residues Y233/Y352 as essential sites for BCR-ABL phosphorylation.
1290
haematologica | 2020; 105(5)