Page 128 - Haematologica May 2020
P. 128
C. Yu et al.
conditional knockout BMDC31 were infected with a BCR- ABL-Cre fusion vector and transplanted into wild-type (wt) recipient mice. Interestingly, deletion of Atg5 was not able to induce a delay in leukemia induction or progres- sion of BCR-ABL transplanted mice (Online Supplementary Figure S1E). Furthermore, Atg5 deletion had no influence on the WBC of the transplanted animals (Online Supplementary Figure S1F), despite efficient deletion of the floxed Atg5 alleles upon Cre expression in BCR-ABL posi- tive BMDC (Online Supplementary Figure S1G).
In order to exclude toxic effects of Beclin-1 knockdown on normal hematopoiesis, we transplanted solely Beclin-1 miR infected BMDC into mice, which exhibited no differ- ences in survival, WBC or lineage phenotype compared to the control group (Online Supplementary Figure S2A-G).
Our results from the in vivo CML mouse model show a
significant and specific impact of Beclin-1 knockdown on CML disease induction.
Active BCR-ABL suppresses autophagy through the BECLIN-1 complex
It has been shown previously that BCR-ABL kinase inhibitors induce autophagy. Accordingly, inhibition of BCR-ABL kinase activity by nilotinib led to an induction of autophagy measured by increased LC3-II expression and punctual LC3 accumulation (Online Supplementary Figure S3A-C). To differentiate, whether the autophagy induction by nilotinib is caused by specific BCR-ABL inhi- bition or due to an unspecific nilotinib effect, we treated nilotinib-resistant Ba/F3-BCR-ABL-T315I cells with nilo- tinib and could show that this treatment failed to induce autophagy, suggesting that active BCR-ABL indeed sup-
ABC
DE
F
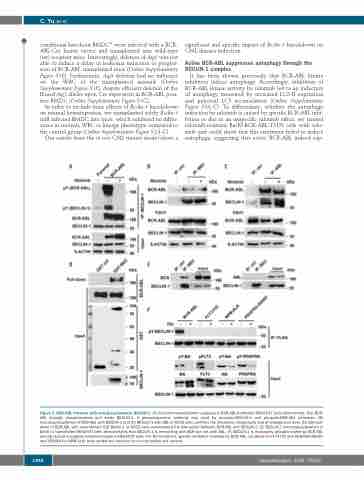
Figure 2. BCR-ABL interacts with and phosphorylates BECLIN-1. (A) Co-immunoprecipitation analyses in BCR-ABL-tranfected HEK293T cells demonstrate, that BCR- ABL strongly phosphorylates and binds BECLIN-1. A phosphotyrosine antibody was used for phospho-BECLIN-1 and phospho-BCR-ABL detection. (B) Immunoprecipitation of BCR-ABL with BECLIN-1 and (C) BECLIN-1 with ABL in K562 cells confirms the interaction reciprocally and at endogenous level. (D) GST-pull- down of BCR-ABL with recombinant GST-Beclin-1 in K562 cells corroborated the interaction between BCR-ABL and BECLIN-1. (E) BECLIN-1 immunoprecipitation in Beclin-1 transfected HEK293T cells demonstrates that BECLIN-1 is interacting with BCR but not with ABL. (F) BECLIN-1 is exclusively phosphorylated by BCR-ABL among several oncogenic tyrosine kinases in HEK293T cells. For TKI treatment, specific inhibitors (nilotinib for BCR-ABL, sorafenib for FLT3-ITD and PDGFRA-D842V, and TAE684 for NPM-ALK) were added into medium four hours before cell harvest.
1288
haematologica | 2020; 105(5)