Page 89 - Haematologica April 2020
P. 89
ARHGEF12 in erythropoiesis of chemotherapy patients
cytology assay by Wright-Giemsa staining showed that the co-injection of stat1a mRNA with arhgef12 MO appeared to promote the immature erythrocyte differenti- ation (Figure 5B). In addition, stat1 MO injection increased gata1 expression (Figure 5C a, a', b, b') but reduced αe1- globin expression (Figure 5C c, c', d, d'), similar to that by arhgef12 MO injection. It is thus likely that STAT1 is involved in the ARHGEF12-p38 MAPK signaling function in erythroid differentiation.
AB
The ARHGEF12-p38 pathway is associated with erythroid regeneration in acute lymphoblastic leukemia patients after chemotherapy
To examine whether ARHGEF12 polymorphism-associ- ated anemia after chemotherapy in ALL patients may engage the p38 pathway, we measured p38 phosphoryla- tion in erythroid cells in seven remission-related BM from ALL patients during maintenance therapy by phospho- flow.30 All seven patient samples with the rs10892563 CC
C
D
E
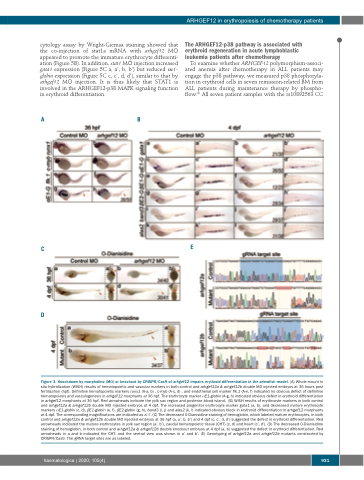
Figure 3. Knockdown by morpholino (MO) or knockout by CRISPR/Cas9 of arhgef12 impairs erythroid differentiation in the zebrafish model. (A) Whole-mount in situ hybridization (WISH) results of hematopoietic and vascular markers in both control and arhgef12a & arhgef12b double MO injected embryos at 36 hours post fertilization (hpf). Definitive hematopoietic markers runx1 (A-a, b) , c-myb (A-c, d) , and endothelial cell marker flk.1 (A-e, f) indicated no obvious defect of definitive hematopoiesis and vasculogenesis in arhgef12 morphants at 36 hpf. The erythrocyte marker αE1-globin (A-g, h) indicated obvious defect in erythroid differentiation in arhgef12 morphants at 36 hpf. Red arrowheads indicate the yolk sac region and posterior blood island. (B) WISH results of erythrocyte markers in both control and arhgef12a & arhgef12b double MO injected embryos at 4 dpf. The increased progenitor erythrocyte marker gata1 (a, b), and decreased mature erythrocyte markers αE1-globin (c, d), βE1-globin (e, f), βE2-globin (g, h), band3 (i, j) and alas2 (k, l) indicated obvious block in erythroid differentiation in arhgef12 morphants at 4 dpf. The corresponding magnifications are indicated as a’-l’. (C) The decreased O-Dianisidine staining of hemoglobin, which labeled mature erythrocytes, in both control and arhgef12a & arhgef12b double MO injected embryos at 36 hpf (a, a’, b, b’) and 4 dpf (c, c’, d, d’) suggested the defect in erythroid differentiation. Red arrowheads indicated the mature erythrocytes in yolk sac region (a’, b’), caudal hematopoietic tissue (CHT) (c, d) and heart (c’, d’). (D) The decreased O-Dianisidine staining of hemoglobin, in both control and arhgef12a & arhgef12b double knockout embryos at 4 dpf (a, b) suggested the defect in erythroid differentiation. Red arrowheads in a and b indicated the CHT, and the ventral view was shown in a’ and b’. (E) Genotyping of arhgef12a and arhgef12b mutants constructed by CRISPR/Cas9. The gRNA target sites are as labeled.
haematologica | 2020; 105(4)
931