Page 289 - Haematologica April 2020
P. 289
mRNA-based therapy for hemophilia A
fer (0.72±0.06 and 0.68±0.08, respectively), suggesting that codon optimization has no beneficial effect on the production of FVIII when cells are transfected with mRNA, in our experimental system.
Systemic delivery of factor VIII-encoding mRNA
to factor VIII-deficient mice leads to the endogenous production of factor VIII
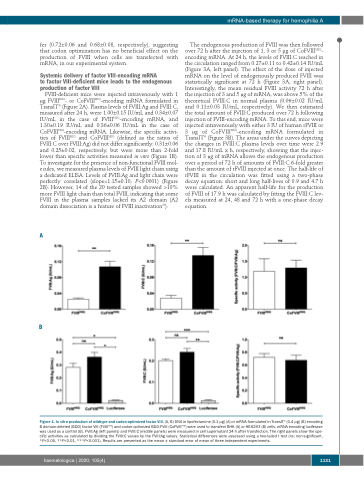
FVIII-deficient mice were injected intravenously with 1 μg FVIIIHSQ- or CoFVIIIHSQ-encoding mRNA formulated in TransIT® (Figure 2A). Plasma levels of FVIII:Ag and FVIII:C, measured after 24 h, were 1.00±0.15 IU/mL and 0.34±0.07 IU/mL in the case of FVIIIHSQ-encoding mRNA, and 1.30±0.19 IU/mL and 0.36±0.06 IU/mL in the case of CoFVIIIHSQ-encoding mRNA. Likewise, the specific activi- ties of FVIIIHSQ and CoFVIIIHSQ (defined as the ratios of FVIII:C over FVIII:Ag) did not differ significantly: 0.31±0.06 and 0.25±0.02, respectively, but were more than 2-fold lower than specific activities measured in vitro (Figure 1B). To investigate for the presence of non-functional FVIII mol- ecules, we measured plasma levels of FVIII light chain using a dedicated ELISA. Levels of FVIII:Ag and light chain were perfectly correlated (slope=1.15±0.10; P<0.0001) (Figure 2B). However, 14 of the 20 tested samples showed >10% more FVIII light chain than total FVIII, indicating that some FVIII in the plasma samples lacked its A2 domain (A2 domain dissociation is a feature of FVIII inactivation26).
A
The endogenous production of FVIII was then followed over 72 h after the injection of 1, 3 or 5 μg of CoFVIIIHSQ- encoding mRNA. At 24 h, the levels of FVIII:C reached in the circulation ranged from 0.27±0.11 to 0.42±0.14 IU/mL (Figure 3A, left panel). The effect of the dose of injected mRNA on the level of endogenously produced FVIII was statistically significant at 72 h (Figure 3A, right panel). Interestingly, the mean residual FVIII activity 72 h after the injection of 3 and 5 μg of mRNA, was above 5% of the theoretical FVIII:C in normal plasma (0.06±0.02 IU/mL and 0.11±0.03 IU/mL, respectively). We then estimated the total amount of FVIII:C produced over 72 h following injection of FVIII-encoding mRNA. To this end, mice were injected intravenously with either 3 IU of human rFVIII or 3 μg of CoFVIIIHSQ-encoding mRNA formulated in TransIT® (Figure 3B). The areas under the curves depicting the changes in FVIII:C plasma levels over time were 2.9 and 17.8 IU/mL x h, respectively, showing that the injec- tion of 3 μg of mRNA allows the endogenous production over a period of 72 h of amounts of FVIII:C 6-fold greater than the amount of rFVIII injected at once. The half-life of rFVIII in the circulation was fitted using a two-phase decay equation: short and long half-lives of 0.9 and 4.7 h were calculated. An apparent half-life for the production of FVIII of 17.9 h was calculated by fitting the FVIII:C lev- els measured at 24, 48 and 72 h with a one-phase decay equation.
B
Figure 1. In vitro production of wildtype and codon-optimized factor VIII. (A, B) DNA in lipofectamine (0.1 μg) (A) or mRNA formulated in TransIT® (0.4 μg) (B) encoding B domain-deleted (BDD) factor VIII (FVIIIHSQ) and codon-optimized BDD-FVIII (CoFVIIIHSQ) were used to transfect BHK (A) or HEK293 (B) cells. mRNA encoding luciferase was used as a control (B). FVIII:Ag (left panels) and FVIII:C (middle panels) were measured in cell supernatant 24 h after transfection. The right panels show the spe- cific activities as calculated by dividing the FVIII:C values by the FVIII:Ag values. Statistical differences were assessed using a two-tailed t test (ns: non-significant, *P<0.05, **P<0.01, ***P<0.001). Results are presented as the mean ± standard error of mean of three independent experiments.
haematologica | 2020; 105(4)
1131