Page 153 - Haematologica April 2020
P. 153
MSC-induced SP phenotype leads to chemoresistance
A
B
C
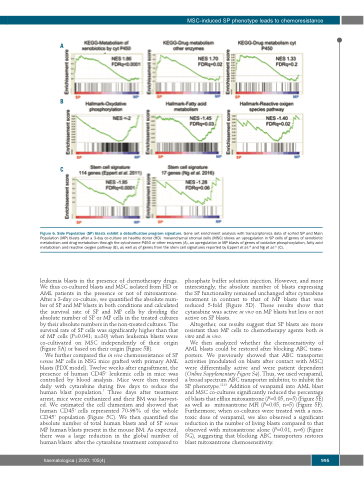
Figure 6. Side Population (SP) blasts exhibit a detoxification program signature. Gene set enrichment analysis with transcriptomics data of sorted SP and Main Population (MP) blasts after a 3-day co-culture on healthy donor (HD) mesenchymal stromal cells (MSC) shows an upregulation in SP cells of genes of xenobiotic metabolism and drug metabolism through the cytochrome P450 or other enzymes (A), an upregulation in MP blasts of genes of oxidative phosphorylation, fatty acid metabolism and reactive oxygen pathway (B), as well as of genes from the stem cell signatures reported by Eppert et al.20 and Ng et al.21 (C).
leukemia blasts in the presence of chemotherapy drugs. We thus co-cultured blasts and MSC isolated from HD or AML patients in the presence or not of mitoxantrone. After a 3-day co-culture, we quantified the absolute num- ber of SP and MP blasts in both conditions and calculated the survival rate of SP and MP cells by dividing the absolute number of SP or MP cells in the treated cultures by their absolute numbers in the non-treated cultures. The survival rate of SP cells was significantly higher than that of MP cells (P=0.041; n=30) when leukemia blasts were co-cultivated on MSC independently of their origin (Figure 5A) or based on their origin (Figure 5B).
We further compared the in vivo chemoresistance of SP versus MP cells in NSG mice grafted with primary AML blasts (PDX model). Twelve weeks after engraftment, the presence of human CD45+ leukemic cells in mice was controlled by blood analysis. Mice were then treated daily with cytarabine during five days to reduce the human blast population.7 Three days after treatment arrest, mice were euthanized and their BM was harvest- ed. We estimated the cell chimerism and showed that human CD45+ cells represented 70-96% of the whole CD45+ population (Figure 5C). We then quantified the absolute number of total human blasts and of SP versus MP human blasts present in the mouse BM. As expected, there was a large reduction in the global number of human blasts after the cytarabine treatment compared to
phosphate buffer solution injection. However, and more interestingly, the absolute number of blasts expressing the SP functionality remained unchanged after cytarabine treatment in contrast to that of MP blasts that was reduced 5-fold (Figure 5D). These results show that cytarabine was active in vivo on MP blasts but less or not active on SP blasts.
Altogether, our results suggest that SP blasts are more resistant than MP cells to chemotherapy agents both in vitro and in vivo.
We then analyzed whether the chemosensitivity of AML blasts could be restored after blocking ABC trans- porters. We previously showed that ABC transporter activities (modulated on blasts after contact with MSC) were differentially active and were patient dependent (Online Supplementary Figure S4). Thus, we used verapamil, a broad spectrum ABC transporter inhibitor, to inhibit the SP phenotype.12,13 Addition of verapamil into AML blast and MSC co-cultures significantly reduced the percentage of blasts that efflux mitoxantrone (P=0.05, n=5) (Figure 5E) as well as mitoxantrone MFI (P=0.05, n=5) (Figure 5F). Furthermore, when co-cultures were treated with a non- toxic dose of verapamil, we also observed a significant reduction in the number of living blasts compared to that observed with mitoxantrone alone (P=0.01, n=6) (Figure 5G), suggesting that blocking ABC transporters restores blast mitoxantrone chemosensitivity.
haematologica | 2020; 105(4)
995