Page 15 - Haematologica April 2020
P. 15
Editorials
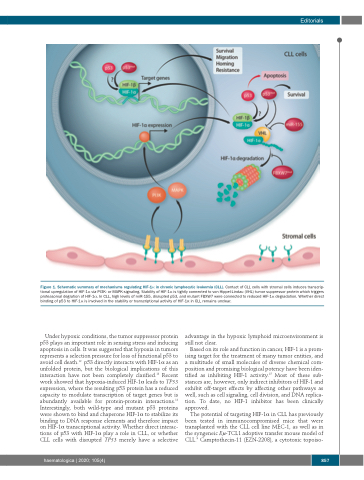
Figure 1. Schematic summary of mechanisms regulating HIF-1α in chronic lymphocytic leukemia (CLL). Contact of CLL cells with stromal cells induces transcrip- tional upregulation of HIF-1α via PI3K- or MAPK-signaling. Stability of HIF-1α is tightly connected to von Hippel-Lindau (VHL) tumor suppressor protein which triggers proteasomal degration of HIF-1α. In CLL, high levels of miR-155, disrupted p53, and mutant FBXW7 were connected to reduced HIF-1α degradation. Whether direct binding of p53 to HIF-1α is involved in the stability or transcriptional activity of HIF-1α in CLL remains unclear.
Under hypoxic conditions, the tumor suppressor protein p53 plays an important role in sensing stress and inducing apoptosis in cells. It was suggested that hypoxia in tumors represents a selection pressure for loss of functional p53 to avoid cell death.12 p53 directly interacts with HIF-1α as an unfolded protein, but the biological implications of this interaction have not been completely clarified.13 Recent work showed that hypoxia-induced HIF-1α leads to TP53 expression, where the resulting p53 protein has a reduced capacity to modulate transcription of target genes but is abundantly available for protein-protein interactions.14 Interestingly, both wild-type and mutant p53 proteins were shown to bind and chaperone HIF-1α to stabilize its binding to DNA response elements and therefore impact on HIF-1α transcriptional activity. Whether direct interac- tions of p53 with HIF-1α play a role in CLL, or whether CLL cells with disrupted TP53 merely have a selective
advantage in the hypoxic lymphoid microenvironment is still not clear.
Based on its role and function in cancer, HIF-1 is a prom- ising target for the treatment of many tumor entities, and a multitude of small molecules of diverse chemical com- position and promising biological potency have been iden- tified as inhibiting HIF-1 activity.15 Most of these sub- stances are, however, only indirect inhibitors of HIF-1 and exhibit off-target effects by affecting other pathways as well, such as cell signaling, cell division, and DNA replica- tion. To date, no HIF-1 inhibitor has been clinically approved.
The potential of targeting HIF-1α in CLL has previously been tested in immunocompromised mice that were transplanted with the CLL cell line MEC-1, as well as in the syngeneic Eμ-TCL1 adoptive transfer mouse model of CLL.9 Camptothecin-11 (EZN-2208), a cytotoxic topoiso-
haematologica | 2020; 105(4)
857