Page 300 - Haematologica March 2020
P. 300
M. Berger et al.
AB
C
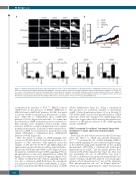
Figure 5. Oxidized low-density lipoproteins induced thrombosis in vivo is prevent by inhibition of phosphodiesterase 3A (PDE3A). Intravital microscopy was per- formed as described in the Online Supplementary Methods. (A) Representative fluorescence images of thrombi formed under different conditions are shown over the course of 40 minutes (min) after vascular injury. Black arrow shows the direction of blood flow. (B) Representative median integrated fluorescence signals of Rhodamine G obtained from an individual carotid thrombus under different conditions. (C) Quantified median integrated fluorescence signals from 10, 20, 30 and 40 min after vascular injury taken from five wild-type (WT) mice for each treatment. *P<0.05; **P<0.01, Kruskal-Wallis Test.
accumulation in response to PGI2 .26,28 OxLDL reduced cAMP levels in the presence of EHNA (2645±122 to 488±7623 fmol cAMP/1x108 platelets; P<0.05), but failed to prevent cAMP accumulation in the presence of milri- none (4761±170 to 4386±15723 fmol cAMP/1x108 platelets; P<0.05 ) (Figure 2Aii and 2Aiii). To confirm that the reduction in cAMP accumulation was not restricted to PGI2, platelets were stimulated with forskolin, which increases cAMP in a receptor-independent non-compart- mentalized mechanism. Forskolin (10 mM)-stimulated ele- vation in cAMP was prevented by preincubation with oxLDL (9506±526 to 4506±1136 fmol cAMP/2x108 platelets; P<0.05) (Figure 2B).
Next, the effects of oxLDL on cAMP signaling were assessed. m (50 nM) induced robust phosphorylation of a number of PKA substrates with apparent molecular weights of: 150, 100, 75, 70, 50, 37 and 20kDa (Figure 2C upper panel), and specifically vasodilator-stimulated phos- phoprotein (VASP) (phosphoVASP-ser157) (Figure 2C mid- dle panel) an established target of PKA signaling.27 These phosphorylation events were diminished by oxLDL (50 mg/mL), but unaffected by nLDL (50 mg/mL) (Figure 2Cii). To further establish that the reduced signaling response was due to enhanced cAMP hydrolysis, the experiment was repeated with 8-CPT-6-Phe-cAMP (50μM), a cell per- meable non-hydrolysable (PDE resistant) analog of cAMP
(Online Supplementary Figure S2). Using a concentration that produced an equivalent quantity of intracellular cAMP (Online Supplementary Figure S3) as m (50 nM), 8- CPT-6-Phe-cAMP caused robust phosphorylation of PKA substrates, which were unaffected by oxLDL (Figure 2D). These data suggest that oxLDL may regulate platelet sen- sitivity to m through modulation of the cAMP-signaling cascade by PDE3A.
CD36 is required for oxidized low-density lipoprotein modulation of cyclic adenosine monophosphate signaling
Previously, we and others have shown that CD36 trans- duces the effects of oxLDL into platelet hyperactivi- ty.14,18,28,29 To examine the role of CD36 in linking oxLDL to altered cAMP signaling, we used a three-pronged strategy: (i) the CD36-blocking antibody FA6-152; (ii) the oxidized phospholipid oxPCCD36, a CD36-specific pathological ligand present in oxLDL,26 and (iii) murine platelets defi- cient in CD36 (Online Supplementary Figure S4). This strat- egy, particularly the use of oxPCCD36, was used to account for differences in human and murine platelet sen- sitivity to human oxLDL. PGI2 induced a robust phospho- rylation of both the preferred ser157 site and the alternative PKA phosphorylation site ser239 in human platelets,30 which was reduced significantly by oxLDL. The presence
814
haematologica | 2020; 105(3)