Page 176 - Haematologica March 2020
P. 176
C. Annageldiyev et al.
E
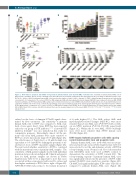
Figure 2. KS99 induces apoptosis and inhibits clonogenicity in primary human acute myeloid (AML) leukemia cells. Sensitivity of primary human AML cells to KS99 (n=21), Cytarabine (Ara-C) (n=13) or ibrutinib (n=9) after 48 hours (h) treatment. (A) Apoptosis was determined as the percentage of Annexin V-positive cells. (B) The IC50 values of KS99 for primary human AML cells, based on the number of viable cells (i.e. Annexin V−/7AAD−). Individual genetic mutations are indicated by colored points. (C) Comparison of IC50 values of KS99 for AML subgroups. AML with myelodysplastic-related changes (MDS-RC) was compared to de novo AML (NPM1 mutant vs. wild type). (D-F) Primary human AML samples and cord blood mononuclear cells (CB MNC) obtained from healthy donors were treated with indicated con- centrations of KS99 and colonies were counted after 10-14 days. Data represent triplicate wells (n=3). (D) Bar graphs show the dose-dependent response of KS99. (E) Bar graph shows a comparison of IC50 values of KS99 for AML patient samples and CB MNC in the colony-forming assay. (F) Representative microscopy images (4X) of AML Patient 1099 colonies after KS99 treatment. Data are the mean±standard error of the mean (SEM) *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; unpaired t-test.
culated on the basis of Annexin-V/7AAD signals deter- mined by flow cytometry. The sensitivity of primary human AML cells to KS99 was compared to AML stan- dard of care agent, Ara-C. Since KS99 has been reported earlier by our group as BTK inhibitor,20,27,28 a known BTK inhibitor, ibrutinib27 was also included in this study for comparative purposes. Interestingly, almost all the pri- mary cells from AML patients were quite sensitive to KS99 at a lower micromolar range (0.3-3 mM) (Figure 2A). The effectiveness of 5 mM Ara-C or 10 mM ibrutinib was equivalent to 0.3-1 mM KS99, defining the potential of rel- atively low doses of KS99 on patient samples (Figure 2A). We next tested whether sensitivity to KS99 is correlated with the mutational status of primary AML samples. The sensitivity of AML patient samples (n=21) with individual mutation status is shown in Figure 2B. Clinical and genetic data for AML patient samples are shown in Online Supplementary Table S1. It is important to note that cases associated with poor prognosis had lower IC50 values and thus higher sensitivity to KS99 (left to right of Figure 2B). NPM1/FLT-3-ITD status seems to be resistant (found in 4
of 6 with highest IC50). The AML subset, AML with myelodysplastic-related changes (MDS-RC), were more sensitive than de novo AML cases (P=0.0077) with or with- out an NPM1 mutation (P=0.012, P=0.045, respectively) (Figure 2C). Within the de novo AML, NPM1 wild-type cases were more sensitive than NPM1 mutant cases (P=0.02) (Figure 2C).
KS99 targets leukemic progenitor cells while sparing normal hematopoietic stem and progenitor cells
Primary human AML cases (n=6) with various cytoge- netic and molecular status (Online Supplementary Table S1) were selected to test the anti-leukemic activity of KS99 in colony-forming assay. Normal cord blood mononuclear cells (CB-MNC) (n=2) were used as HSPC controls to demonstrate the selectivity of KS99 towards LSC. The colony-forming capacity of primary human AML cases was significantly reduced in the presence of KS99, while normal CB-MNC were much less sensitive (Figure 2D). CB-MNC had significantly higher IC50 values (4.2-fold) as compared to primary human AML cases (Figure 2E).
690
haematologica | 2020; 105(3)
A
B
CD
F