Page 93 - 2020_02-Haematologica-web
P. 93
Irf2bp2b regulates zebrafish NMP cell fate choice
functional divergence occurred early in vertebrate evolu- tion.26
The zebrafish is an excellent model organism for the study of hematopoiesis.27 Like mammalian hematopoiesis, zebrafish hematopoiesis also consists of primitive and definitive waves which emerge sequentially in distinct anatomical sites.
Human IRF2BP2 mRNA is distributed in dozens of tis- sues, with the most prominent expression being found in bone marrow (https://www.ncbi.nlm.nih.gov/gene/359948). Zebrafish irf2bp2b is also ubiquitously expressed in devel- oping embryos. irf2bp2b transcript was detected in the green fluorescent protein (GFP)-positive cells enriched from Tg(gata1:eGFP), Tg(pu.1:eGFP), Tg(mpx:eGFP), and Tg(mpeg1.1:eGFP) embryos (Online Supplementary Figure S2). To evaluate the effects of irf2bp2b on hematopoietic differentiation and lineage commitment, a mutant line was generated using the CRISPR/Cas9 system targeting the first exon of the irf2bp2b gene and introducing a 26 nt dele- tion which results in a truncated protein by frameshifting
A
B
CD
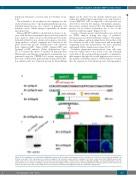
(Figure 1A, B). Moreover, the mutant irf2bp2b gene was cloned into an HA-tagged expressing vector and transfect- ed into HEK293T cells. As expected, a short protein was detected by western blot analysis. Meanwhile, immuno- fluorescence analysis showed that this Irf2bp2b mutant protein lost its nuclear localization due to loss of the nuclear localization signal28 (Figure 1C, D).
A series of hematopoietic-related markers was detected by WISH analysis during the stage of primitive hematopoiesis in irf2bp2b-defecient embryos. The primi- tive macrophages and neutrophils derived from the rostral blood island, as well as the erythrocytes and neutrophils originating from the intermediate cell mass remained unchanged (Online Supplementary Figure S3A-L, W).
Definitive pluripotent hematopoietic stem cells arise from the ventral wall of the dorsal aorta, the zebrafish equivalent of the aorta/gonad/mesonephros of mammals, then migrate through the caudal hematopoietic tissue to the thymus and kidney marrow. WISH analyses revealed that the expression of the hematopoietic stem/progenitor
Figure 1. The establishment of a zebrafish irf2bp2b knockout line. (A) Schematic representation of the Cas9 target site in the first exon of zebrafish irf2bp2b. The deleted nucleotides in the mutant gene are marked by hyphens. (B) Schematic representation of wildtype (501 amino acids) and mutant Irf2bp2b proteins (201 amino acids). The site where the frameshift was introduced is marked by triangles. (C) Western blot analysis of HA-tagged wildtype and mutant Irf2bp2b proteins. (D) Immunofluorescence analysis of wildtype (top panel) and mutant Irf2bp2b (bottom panel) proteins, demonstrating that the truncated protein lost its nuclear local- ization. AA: amino acids; mut: mutated; WB: western blot; HA: human influenza hemagglutinin; DAPI: 4′,6-diamidino-2-phenylindole.
haematologica | 2020; 105(2)
327