Page 45 - 2020_02-Haematologica-web
P. 45
Innate immune cells in sickle cell disease
Table 1. The main potential therapeutic agents targeting innate immune cells in sickle cell disease.
Therapeutic agent
Hydroxyurea
Crizanlizumab (SEG101)
Rivipansel (GMI-1070)
Sevuparin
IVIG
NKTT120
Ticagrelor
Targeted innate immune cells
Neutrophils, eosinophils, monocytes, NK cells, platelets
Neutrophils, platelets
Neutrophils, platelets
Neutrophils, platelets
Neutrophils
iNKT cells
Platelets
Mechanism of action
Multimodal mechanism including myelosuppression
P-selectin inhibitor
(monoclonal antibody) Pan-selectin inhibitor
Multimodal mechanism including
P- and L-selectin inhibition
Inhibits neutrophil adhesion and RBC-neutrophil interactions
iNKT cell depletion
(monoclonal antibody)
ADP receptor antagonist
Study ID #
Phase
FDA-approved III
NCT03814746 III
FDA-approved
NCT02187003 III
NCT02515838 II
NCT01757418 II
NCT01783691 I
NCT03615924 III
ID #: identification number; NK cells: natural killer cells; FDA: US Food and Drug Administration; IVIG: intravenous immunoglobulin; RBC: red blood cell; iNKT cells: invariant nat- ural killer T cells; ADP: adenosine diphosphate.
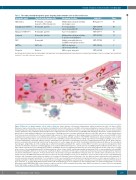
Figure 4. Main roles of innate immune cells in sickle cell disease. Innate immune cells promote inflammation, adhesion and pain in sickle cell disease (SCD). Monocytes may be activated by several mechanisms, including interactions with red blood cells (RBC), reticulocytes and platelets, as well as toll-like receptor 4 (TLR4) stimulation by heme and lipopolysaccharide. Activated monocytes produce pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor-α, which activate endothelial cells, resulting in enhanced expression of adhesion molecules. The interaction of sickle RBC with endothelial cells induces cellular oxidant stress, which leads to transendothelial migration of blood monocytes. Patrolling monocytes scavenge endothelium-adherent sickle RBC and take up cellular debris derived from heme-exposed endothelial cells, thereby leading to high expression of heme oxygenase-1. Macrophages may be activated by heme, released from RBC in SCD, resulting in increased production of pro-inflammatory cytokines, especially IL-1β, through activation of the NLRP3 inflammasome. Elevated plasma levels of sphin- gosine-1-phosphate (S1P) induce IL-6 expression via S1P receptor 1 (S1PR1), and IL-6 in turn promotes S1PR1 expression by macrophages, leading to a vicious cycle of chronic inflammation. Neutrophil adhesion involves endothelial P-selectin, which is upregulated in response to TLR4 activation by heme in SCD. Heme may also promote neutrophil extracellular trap formation via the generation of reactive oxygen species (ROS) in neutrophils. Endothelial E-selectin induces the clustering of macrophage-1 antigen (Mac-1) on the leading edge of adherent neutrophils, allowing for the capture of sickle RBC. P-selectin is also expressed by platelets, which promotes the formation of platelet-neutrophil aggregates. The platelet NLRP3 inflammasome is activated in SCD, via HMGB1/TLR4 and Bruton tyrosine kinase, lead- ing to enhanced production of pro-inflammatory cytokines, including IL-1β. Increased platelet activation may also result from depletion of nitric oxide (NO), secondary to the release of free hemoglobin from RBC. Activated platelets release soluble CD40L and thrombospondin, which binds CD36 on both endothelial cells and RBC, thereby promoting RBC adhesion to microvascular endothelium. Mast cell activation in SCD may be mediated by ischemia-reperfusion, hemolysis with NO depletion, and chronic inflammation. Substance P released from mast cells promotes neurogenic inflammation and pain, but it also activates mast cells themselves via neu- rokinin 1 receptor and MAS-related G-protein-coupled receptor X2, thereby inducing a vicious cycle of substance P release as well as the release of pro-inflammatory cytokines and chemokines, which promotes immune cell recruitment. Histamine released from mast cells also induces plasma extravasation and endothelial P- selectin expression. Invariant natural killer T-cell activation in SCD is associated with increased interferon-γ production and A2A receptor expression. Adhesion of cir- culating eosinophils to fibronectin in SCD is mediated by several integrins, including Mac-1, and activated eosinophils are responsible for enhanced ROS production. A2AR: adenosine A2A receptor; BTK: Bruton tyrosine kinase; Hb: hemoglobin; HO-1: heme oxygenase-1; IFN: interferon; iNKT cell: invariant natural killer T cell; LPS: lipopolysaccharide; MRGPRX2: MAS-related G-protein-coupled receptor X2; NET: neutrophil extracellular traps; NK1R: neurokinin 1 receptor; PlGF: placental growth factor; TLR: toll-like receptor 4; TNF-α: tumor necrosis factor alpha.
haematologica | 2020; 105(2)
279