Page 44 - 2020_02-Haematologica-web
P. 44
S. Allali et al.
the same line of evidence, crossing SCD mice with lym- phocyte-deficient mice resulted in decreased pulmonary dysfunction, whereas the adoptive transfer of iNKT cells reconstituted injury.
In SCD mice, pulmonary iNKT-cell activation was asso- ciated with a nine-fold increase in A2AR mRNA level as compared with the level in control mice. Treating SCD mice with an A2AR agonist improved pulmonary inflam- mation and prevented further hypoxia-reoxygenation– induced lung injury by inhibiting iNKT-cell activation.69 In SCD patients, A2AR was induced during VOC in CD4+ but not CD4- iNKT cells.72 This induction may serve to inhibit iNKT-cell activation over time in a counter-regulatory mechanism, thereby limiting the extent of the inflamma- tory immune response. A2AR transcription seems to be induced by NF-κB activation because the use of NF-κB inhibitors in cultured human iNKT cells blocked the induction of A2AR mRNA and protein.72 Furthermore, iNKT cells from SCD patients showed concomitant high expression of A2AR and CD39, the ecto-ATPase that con- verts ATP and ADP to AMP, thereby resulting in increased adenosine production, which limits iNKT-cell activation.73 In a phase I trial of the A2AR agonist regadenoson in 27 adult SCD patients, iNKT-cell activation, measured by phospho-NF-κB, IFN-γ and A2AR expression, was increased in patients as compared with controls and during VOC as compared with steady-state.74 A 24-hour infusion of regadenoson during VOC decreased the activation of
iNKT cells by 50%, to levels similar to those in steady- state patients and in controls, without any reported toxic- ity. However, in a phase II, randomized, placebo-con- trolled trial of 92 SCD patients, a 48-hour infusion of regadenoson at the same dose during VOC did not signif- icantly decrease iNKT-cell activation or the severity of the crises.75
Another approach based on iNKT-specific depletion with the humanized IgG1κ monoclonal antibody NKTT120 is currently under investigation, and it has already been shown that a single intravenous bolus pro- duces rapid, specific and sustained iNKT-cell depletion, thereby allowing for a regimen of infusion every 3 months.76 Because iNKT cells play important roles in immunity, there are legitimate concerns about a possible increase in susceptibility to infections, but no adverse effect was reported in a first multicenter, single-ascending- dose trial designed to determine the pharmacokinetics, pharmacodynamics and safety in SCD patients in steady- state. The next step should be a randomized, double- blind, placebo-controlled trial to assess the efficacy of NKTT120 in preventing VOC occurrence.
Platelets
Platelets are essential in hemostasis and thrombosis, but they are also important mediators of vascular inflamma- tion. Platelets were found to be activated in SCD patients in steady-state and even more so during VOC.8,77 Platelet
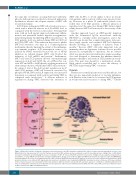
Figure 3. Mast cells in sickle cell disease. Histamine released from mast cells (MC) stimulates endothelial H2 and H4 receptors, thereby inducing the release of von Willebrand factor and expression of P-selectin. Tryptase released from MC activates protease-activated receptor 2 on peripheral nerve endings, thus contributing to nociceptor sensitization and stimulating the release of substance P (SP). SP released from MC and from sensory nerve endings increases plasma extravasation via neurokinin 1 receptor (NK1R) and promotes neurogenic inflammation. SP also acts on MC via NK1R and MAS-related G-protein-coupled receptor X2 (MRGPRX2), thus inducing more SP release in an amplification loop of MC activation. MRGPRX2 stimulation by SP induces the release of several cytokines and chemokines, which promotes immune cell recruitment. MC degranulation in response to morphine is also mediated by MRGPRX2. Hemolysis in sickle cell disease (SCD) may contribute to MC activation because it is responsible for nitric oxide depletion, which is known to activate MC. MC activation appears to contribute to endothelial dysfunction in SCD, via endoplasmic reticulum stress-mediated P-selectin expression and increased endothelial permeability. NO: nitric oxide; PAR-2: protease-activated receptor 2; RBC: red blood cell; VWF: von Willebrand factor.
278
haematologica | 2020; 105(2)