Page 70 - 2019_12-Haematologica-web
P. 70
T. Raskovalova et al.
diagnosis were: 1) the presence of ≥10% dysplastic cells in any hematopoietic lineage; 2) the exclusion of acute myeloid leukemia (defined by the presence of ≥20% PB or BM blasts); and 3) the exclusion of reactive etiologies of cytopenia and dysplasia.
Consistent with the World Health Organization (WHO) classification,1 MDS subcategorization was based on the degree of dysplasia (unilineage vs. multilineage), blast per- centages, presence of ring sideroblasts, and cytogenetic analysis (del(5q)). The criteria for CMML diagnosis were: 1) the presence of persistent PB monocytosis ≥1x109/L; and 2) monocytes accounting for more than 10% of the white blood cell differential count.1 Idiopathic cytopenia of uncertain significance (ICUS) was defined by unex- plained mild persistent cytopenia for 4-6 months and the failure to establish the diagnosis of MDS according to the guidelines.5,21-23
In the retrospective case control study, the reference standard was available for MDS cases only and no control subject received cytomorphological evaluations. In con- trast, the reference standard was available for all patients enrolled in the prospective validation cohort study.
Sample size
Assuming an area under the receiver operating charac- teristic (ROC) curve point estimate of 0.95, we estimated
that a sample size of 88 participants (comprising 44 MDS patients and 44 controls) would provide a precision of ±0.05 [95% confidence interval (CI) ranging from 0.90 to 1.00].24
Precision and reproducibility assessment
We evaluated intra- and inter-assay precision, repro- ducibility between study sites, and specimen stability for RCV measurements of MPO expression in the PB neu- trophil population according to current guidelines.25-27
Statistical analysis
We assessed the independent associations of MDS with RCV for neutrophil MPO expression measured by flow cytometric analysis in PB, using multivariable logistic regression. Odds ratio estimates were adjusted for age and baseline characteristics that were significantly associated with MDS in univariable analysis [C-reactive protein (P<0.001) and creatinine (P=0.03) concentrations]. Because hemoglobin concentration, platelet count, and absolute neutrophil count were part of the MDS definition, they were not entered as co-variates in the multivariable model. Twenty-one observations were imputed because of missing values for C-reactive protein and/or creatinine concentrations. Additional variables entered in the impu- tation model included age, gender, RCV, and MDS diagno-
ABCD
EFGH
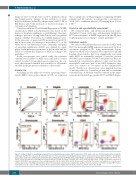
Figure 1. Gating strategy for quantifying peripheral blood neutrophil myeloperoxidase (MPO) expression. CD45+ viable cells were first individualized by crossing the singlet gate (A), FSC-SSC leukocytes (B), and CD45+ gate (C). Three populations including granulocytes (CD15+ CD14–), monocytes (CD14+ CD15low/–), and lympho- cytes (CD15– CD14–) were identified (D). Eosinophils were individualized by CD45high CD16 low (E). Mature neutrophils were individualized by Boolean intersection: [CD15+ CD14–] (D) AND NOT [CD45high CD16 low] (E) AND NOT [CD14+ CD15low/–] (D) AND NOT [CD15- CD14-] (D) AND [CD16+ CD11b+] (F). Robust coefficient of variation (RCV) MPO was evaluated on the resulting population (G). The CD16 CD64 dot plot (H) was used to verify that the mature neutrophils were correctly select- ed: they appeared as CD16high and CD64low cluster. The populations identified were lymphocytes (purple), monocytes (green), eosinophils (orange), MPO mature neutrophils (red). CD: cluster of differentiation; FSC-H: forward scatter height; FSC-A: forward scatter area; SSC-H: side scatter height.
2384
haematologica | 2019; 104(12)