Page 93 - 2019_10 resto del Mondo_web
P. 93
Tmem30a in erythropoiesis and EPOR signaling
and EPOR signaling, we analyzed the lipid raft distribu- tion by using cholera toxin subunit B (CTxB) to label endogenous GM1 ganglioside, a component of lipid rafts. Co-staining of GM1 and EPOR on Ter119low erythroid cells showed that EPO treatment stimulated the EPOR cluster- ing and co-localized with the lipid rafts. Interestingly, Tmem30a deletion inhibited lipid raft clustering and EPOR co-localization with the lipid rafts (Figure 5A and B). The specificity of the EPOR antibody was demonstrated by immunofluorescence analysis omitting the primary anti- EPOR antibody as negative control (Online Supplementary Figure S5A). To further determine whether TMEM30A deficiency impedes the recruitment of EPOR to lipid raft, lipid rafts were separated from TER119 negative fetal liver cells upon EPO treatment. Western blot showed that the EPOR protein was presented in the extracted lipid rafts, but the level of EPOR was decreased in cKO cells (Online Supplementary Figure S5B). Taken together, these data sug- gest that the impaired lipid raft clustering upon EPO stim- ulation in Tmem30a-deficient fetal liver erythroid cells may compromise the EPO/EPOR signaling, which is essential for fetal liver erythropoiesis.
Tmem30a deficiency compromises STAT5 activation and down-regulates pro-survival protein BCL-XL
It has been well documented that EPO/EPOR signaling
activates the JAK2-STAT5 pathway to sustain the viability of erythroid cells in the fetal liver.13 The above findings strongly suggest that the observed phenotypic changes of Tmem30a knockdown erythroid cells may be due to impaired EPO/EPOR signal transduction. To test this, we investigated the key components of the EPO/EPOR-JAK2- STAT5 signaling pathway. FACS analysis using EPOR anti- body staining on living TER119– erythroid cells showed that the levels of EPOR expression on the cell surface were not reduced in cKO mice compared to control mice (Online Supplementary Figure S5C). Intriguingly, after EPO stimula- tion in culture, the activation of the JAK2-STAT5 signaling pathway was significantly impaired in Tmem30a deficient fetal liver cells, as demonstrated by the lack of phosphory- lation of STAT5 in cKO cells upon EPO treatment (Figure 6A). In addition, the downstream transcriptional target genes of STAT5 signaling28 such as Pim1, Socs3 and BCL- XL were significantly decreased after EPO exposure in Tmem30a deficient fetal livers compared to the controls (Figure 6B). Among these genes, BCL-XL is essential for the survival of erythroid cells. Therefore, we further analyzed the protein levels of BCL-XL by western blotting. The pro- survival protein BCL-XL was dramatically decreased in cKO fetal liver although exposure to EPO did not increase BCL- XL expression (Figure 6C). Perhaps 30-min exposure to EPO was not enough to increase the Bcl-XL protein level. BCL-
AB
C
D
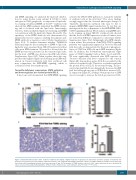
Figure 3. Tmem30a-deficient mice (cKO) fetal liver erythroid cells display a higher frequency of apoptosis. (A and B) Flow cytometric analysis of Tmem30a cKO fetal livers revealed increased percentages of Annexin V positive cells compared with wild-type littermate controls. (C) TUNEL staining of paraffin sections from fetal livers fixed in 4% formaldehyde solution. Scale bars represent 50 μm. (D) The number of TUNEL positive cells per 1.3*105 square micrometers were calculated. All values are presented as Mean±Standard Error of Mean of three fetal livers per each embryonic data. *P<0.05; *** P<0.001.
haematologica | 2019; 104(10)
1989