Page 17 - 2019_09-HaematologicaMondo-web
P. 17
Editorials
tion and subsequent degradation of BCR-ABL. Furthermore, expression of transcriptional factor MYC plays a critical role in the proliferation and self-renewal of leukemic stem cells. Reavie et al. demonstrated that the E3 ubiquitin ligase FBW7 is required for the survival and maintenance of BCR-ABL+ leukemia initiating cells (LIC) by modifying the expression of MYC through FBW7- mediated ubiquitination and degradation.15 Deletion of Fbw7 leads to c-Myc overexpression, p53-dependent LIC- specific apoptosis, and the eventual inhibition of tumor progression. A decrease in either c-Myc protein levels or attenuation of the p53 response rescues LIC activity and disease progression. UBE2A acts as E2 conjugating enzyme for FBW7, and mutations in UBE2A attenuate FBW7 activity and maintain the basal expression level of
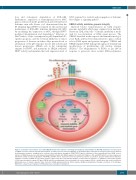
MYC required for survival and propagation of leukemic blast (Figure 1, signaling path B).
UBE2A activity maintains genomic integrity
Myeloid blastic transformation in CML requires genomic instability which may originate from imatinib- refractory CML stem cells.16 Genomic instability is medi- ated by loss-of-function of DNA repair process. The UBE2A described in this report is the human homolog of yeast Rad6, and has been demonstrated to play a critical role in DNA repair and genome integrity.17 UBE2A and UBE2B regulate DNA damage through post-translational modification of proliferating cell nuclear antigen (PCNA).18 The ubiquitination of PCNA at Lys 164 in response to genotoxic stress recruits DNA polymerase
Figure 1. Schematic representation of possible UBE2A-mediated mechanisms controlling myeloid blastic transformation in BCR-ABL leukemias. Possible targets of UBE2A relevant to leukemic myeloid transformation. (A and B) Cytosolic functions. UBE2A is an E2 ubiquitin ligase important in emergency myelopoiesis induced by inflammatory cytokines, abundant in the leukemic microenvironment, through the TRAF/TRIF E3 ligases, regulators of the transcriptional factor NFkB. Loss-of- function of UBE2A may associate with impaired myeloid differentiation. (B) UBE2A regulates the activity of E3 ligases c-CBL and FBW7, which are tumor suppressors with known activity to induce degradation of BCR-ABL and MYC, whose expression in turn is required for leukemic acceleration. Question marks denote that these pathways of activity of UBE2A are speculative and not supported by direct experimental designs. (C and D) Nuclear functions. (C) UBE2A is a well known regulator of DNA repair through its cognate E3 ligase RAD18, which monoubiquitinates the proliferating cell nuclear antigen (PCNA), a modification that recruits translesion DNA polymerases to stalled replication forks. (D) Active UBE2A (phosphorylated by CDK9) regulates H2Bmonoubiquitination through recruitment of the E3 ligase RNF20/40, a major step in regulation of RNA polymerase II and gene transcription.
haematologica | 2019; 104(9)
1695