Page 130 - 2019_09-HaematologicaMondo-web
P. 130
E.C. Matheson et al.
AB
CD
EF
G
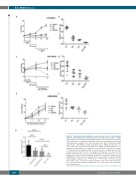
Figure 2. Selumetinib and dexamethasone show synergy in vivo in RAS pathway- mutated acute lymphoblastic leukemia. (A-F) In vivo drug efficacy studies of single drugs and their combination in RAS pathway mutated-acute lymphoblastic leukemia (ALL) showing dose scheduling and peripheral blood monitoring before and during dosing and spleen weights at the end of dosing for mice with L779-NRAS (A and B, respectively), L897-KRAS (C and D) and L829 relapse- KRAS (E and F) ALL. For L779, mice were dosed with selumetinib at 25 mg/kg and dexamethasone at 1 mg/kg twice daily and then once daily after a recovery period. For L897, the dosage of selumetinib was 25 mg/kg and that of dexamethasone 0.5 mg/kg (bid), with the dexamethasone being increased to 1 mg/kg (sid) following a recovery period. For L829R, selumetinib was dosed at 25 mg/kg (bid) and dexamethasone at 0.25 mg/kg (sid). (G) The mean and standard deviation are shown for combined spleen weight data for all three efficacy experiments (one-way analysis of variance with the Tukey multiple comparison test, ***P<0.001, ****P<0.0001; n=17 mice treated with control vehicle, n=17 treated with selumetinib, n=15 treated with dexametha- sone and n=14 treated with the combination. CV: control vehicle; Sel: selumetinib; Dex: dexamethasone.
1808
haematologica | 2019; 104(9)