Page 28 - 2019_02-Haematologica-web
P. 28
L. Prieto-Torres et al.
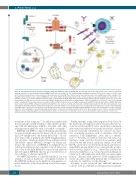
Figure 4. New targeted therapies in advanced primary cutaneous anaplastic large cell lymphoma. Brentuximab vedotin is a drug composed of a chimeric anti-CD30 antibody linked to four monomethyl auristatin (MMAE) molecules (on average) (1). This antibody-drug conjugate first binds to CD30 on the surface of pcALCL cells (2). It is then internalized by a receptor-mediated endocytosis process (3 and 4). The resultant vesicle fuses with lysosomes (4), leading to proteolytic cleavage of the dipeptide linker and the presence of free MMAE molecules, which inhibit tubulin polymerization of the cellular cytoskeleton and arrest growth of pcALCL tumor cells. Gamma-secretase (γ-secretase) inhibitors prevent release of intracellular NOTCH1 (ICN1) from membrane-tethered heterodimeric NOTCH1 protein. This causes the downregulation of the tumor cell nuclear factor-kB (NFKB) pathway and the inactivation of survival genes. JAK1/2/3 inhibitors are effective in vitro for controlling pcALCL cell growth. This mechanism involves oncogenic JAK1 or STAT3 mutations associated with the hyperactive pSTAT3 shown in pcALCL with an NPM1-TYK2 gene fusion and oncogenic STAT3 activation. In addition, anti-ALK molecules such as crizotinib, alectinib, and ceritinib in pcALCL patients with ALK rearrangements could downregulate the STAT3 pathway, ultimately inducing tumor-cell death. IPH4102 is a humanized monoclonal antibody directed against the cellular receptor KIR3DL2 (CD158K). This receptor has been shown to be aberrantly expressed in advanced pcALCL. IPH4140 targets KIR3DL2 in tumor cells and promotes cell lysis after link- ing to the CD16 activating receptor through antibody-dependent, cell-derived cytotoxicity mediated by NK cells. At the epigenetic level, histone deacetylase (HDAC) inhibitors and demethylating agents have demonstrated a degree of effectiveness at inducing cell-cycle arrest, differentiation and/or apoptosis of tumor cells.
treatment of this subgroup.68,103 In addition, patients with the rare subtype of ALK+ pcALCL could benefit from ther- apy with the ALK kinase inhibitor crizotinib, and other new-generation inhibitors such as alectinib or ceritinib.104
KIR3DL2 (CD158K) is a killer cell immunoglobulin-like receptor normally expressed by minor subsets of circulat- ing CD8+ lymphocytes and natural killer cells.105 In con- trast, tumor cells of pcALCL and CD30+ lymphoprolifera- tive disorders, which are derived from cell lines Mac2a and Mac2b, express KIR3DL2 strongly.105 In Sézary syn- drome, the expression of KIR3DL2 in malignant blood cells has been proposed as a marker of blood tumor bur- den.106 The novel agent IPH4102 is a monoclonal antibody directed against KIR3DL2 that has proven effective in in vitro cell lines of advanced pcALCL.47
The NOTCH pathway is activated in pcALCL as a con- sequence of various mutations.89 Deregulated activity of the NOTCH pathway can be inhibited using gamma sec- retase inhibitors, as has been shown in an in vitro experi- ment.89
Finally, therapies acting at the epigenetic level, based on the mutations and epigenetic alterations described above, could be useful in pcALCL. More specifically, romidepsin and vorinostat, inhibitors of histone deacetylase, derived from the bacterium Chromobacterium violaceum are effec- tive at inducing apoptosis with an antitumor effect in cutaneous T-cell lymphomas, alone and in combina- tion.107,108 Recently, researchers from the Yale School of Medicine, using cell lines derived from patients with advanced MF and Sézary syndrome (MyLa, Sez4, HH, Hut78), observed in vitro that bromodomain and extrater- minal (BET) protein inhibitors are synergistically potenti- ated by BCL2 inhibitors (e.g., venetoclax) and histone deacetylase inhibitors (e.g., vorinostat and romidepsin) in cutaneous T-cell lymphomas with MYC oncogene ampli- fication.109 Patients with cutaneous CD30+ lymphoprolif- erative disorders with MYC-induced Id2 overexpression could also be candidates for this therapy.24
Recently, it was proposed that miR-155 inhibition could be used in combination with apoptotic treatments
232
haematologica | 2019; 104(2)